Abstract
Background
Pancreatic stellate cells (PSCs) play a pivotal role in pancreatic fibrosis, a characteristic feature of pancreatic cancer. Although it is still controversial, previous studies have suggested that PSCs promote the progression of pancreatic cancer by regulating the cell functions of cancer cells. PSCs produce large amounts of IL-6, which promotes the accumulation of myeloid-derived suppressor cells via a signal transducers and activator of transcription 3 (STAT3)-dependent mechanism. But the role of IL-6/STAT3 pathway in the interaction between PSCs and pancreatic cancer cells remains largely unknown.
Aims
To clarify the role of IL-6/STAT3 in the interaction between PSCs and cancer cells.
Methods
Human pancreatic cancer cells (Panc-1 and SUIT-2 cells) were treated with conditioned medium of immortalized human PSCs (PSC-CM). The effects of PSC-CM and IL-6 neutralization on the mRNA expression profiles were examined using Agilent’s microarray. Activation of STAT3 was assessed by Western blotting using an anti-phospho-specific antibody. Cellular migration was examined by a two-chamber assay. The expression of markers related to epithelial–mesenchymal transition (EMT) was assessed by real-time reverse transcription PCR.
Results
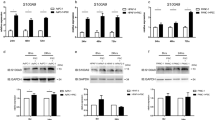
PSC-CM induced the activation of STAT3 in pancreatic cancer cells. Neutralization of IL-6 suppressed the PSC-CM-induced upregulation of genes including complement factor B, lipocalin, and chemokine (C–C motif) ligand 20. Inhibition of IL-6/STAT3 pathway by anti-IL-6 antibody or a STAT3 inhibitor (NSC74859) inhibited the PSC-CM-induced migration and the expression of EMT-related markers (Snail and cadherin-2) in pancreatic cancer cells.
Conclusion
IL-6/STAT3 pathway regulates the PSC-induced EMT and alterations in gene expression in pancreatic cancer cells.








Similar content being viewed by others
Abbreviations
- CFB:
-
Complement factor B
- CCL20:
-
Chemokine (C–C motif) ligand 20
- CM:
-
Conditioned medium
- DAPI:
-
4′,6-diamidino-2-phenylindole
- EMT:
-
Epithelial–mesenchymal transition
- ERK:
-
Extracellular signal-regulated kinase
- GFAP:
-
Glial fibrillary acidic protein
- IκB:
-
Inhibitor of κB
- IPA:
-
Ingenuity Pathway Analysis
- JNK:
-
c-Jun N-terminal kinase
- LCN2:
-
Lipocalin
- MAPK:
-
Mitogen-activated protein kinase
- PSCs:
-
Pancreatic stellate cells
- RT:
-
Reverse transcription
- SMA:
-
Smooth muscle actin
- STAT:
-
Signal transducers and activator of transcription
References
Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617.
Neesse A, Michl P, Frese KK, et al. Stromal biology and therapy in pancreatic cancer. Gut. 2011;60:861–868.
Apte MV, Haber PS, Applegate TL, et al. Periacinar stellate shaped cells in rat pancreas: identification, isolation and culture. Gut. 1998;43:128–133.
Erkan M, Adler G, Apte MV, et al. StellaTUM: current consensus and discussion on pancreatic stellate cell research. Gut. 2012;61:172–178.
Masamune A, Shimosegawa T. Signal transduction in pancreatic stellate cells. J Gastroenterol. 2009;44:249–260.
Apte MV, Wilson JS, Lugea A, Pandol SJ. A starring role for stellate cells in the pancreatic cancer microenvironment. Gastroenterology. 2013;144:1210–1219.
Masamune A, Shimosegawa T. Pancreatic stellate cells-multi-functional cells in the pancreas. Pancreatology. 2013;13:102–105.
Bachem MG, Schünemann M, Ramadani M, et al. Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology. 2005;128:907–921.
Hwang RF, Moore T, Arumugam T, et al. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res. 2008;68:918–926.
Vonlaufen A, Joshi S, Qu C, et al. Pancreatic stellate cells: partners in crime with pancreatic cancer cells. Cancer Res. 2008;68:2085–2093.
Kikuta K, Masamune A, Watanabe T, et al. Pancreatic stellate cells promote epithelial–mesenchymal transition in pancreatic cancer cells. Biochem Biophys Res Commun. 2010;403:380–384.
Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial–mesenchymal transitions in development and disease. Cell. 2009;139:871–890.
Lunardi S, Jamieson NB, Lim SY, et al. IP-10/CXCL10 induction in human pancreatic cancer stroma influences lymphocytes recruitment and correlates with poor survival. Oncotarget. 2014;5:11064–11080.
Mace TA, Ameen Z, Collins A, et al. Pancreatic cancer-associated stellate cells promote differentiation of myeloid-derived suppressor cells in a STAT3-dependent manner. Cancer Res. 2013;73:3007–3018.
Hamada S, Masamune A, Takikawa T, et al. Pancreatic stellate cells enhance stem cell-like phenotypes in pancreatic cancer cells. Biochem Biophys Res Commun. 2012;421:349–354.
Masamune A, Kikuta K, Watanabe T, et al. Fibrinogen induces cytokine and collagen production in pancreatic stellate cells. Gut. 2009;58:550–559.
Siddiquee K, Zhang S, Guida WC, et al. Selective chemical probe inhibitor of Stat3, identified through structure-based virtual screening, induces antitumor activity. Proc Natl Acad Sci USA. 2007;104:7391–7396.
Masamune A, Watanabe T, Kikuta K, et al. Nuclear expression of interleukin-33 in pancreatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2010;299:G821–G832.
Hamada S, Masamune A, Miura S, Satoh K, Shimosegawa T. MiR-365 induces gemcitabine resistance in pancreatic cancer cells by targeting the adaptor protein SHC1 and pro-apoptotic regulator BAX. Cell Signal. 2014;26:179–185.
Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19:185–193.
Quackenbush J. Microarray data normalization and transformation. Nat Genet. 2002;32(Suppl):496–501.
Takikawa T, Masamune A, Hamada S, et al. miR-210 regulates the interaction between pancreatic cancer cells and stellate cells. Biochem Biophys Res Commun. 2013;437:433–439.
Garbers C, Aparicio-Siegmund S, Rose-John S. The IL-6/gp130/STAT3 signaling axis: recent advances towards specific inhibition. Curr Opin Immunol. 2015;34:75–82.
Lee MJ, Na K, Jeong SK, et al. Identification of human complement factor B as a novel biomarker candidate for pancreatic ductal adenocarcinoma. J Proteome Res. 2014;13:4878–4888.
Klemm C, Dommisch H, Göke F, et al. Expression profiles for 14-3-3 zeta and CCL20 in pancreatic cancer and chronic pancreatitis. Pathol Res Pract. 2014;210:335–341.
Leung L, Radulovich N, Zhu CQ, et al. Lipocalin2 promotes invasion, tumorigenicity and gemcitabine resistance in pancreatic ductal adenocarcinoma. PLoS One. 2012;7:e46677.
Beigel F, Friedrich M, Probst C, et al. Oncostatin M mediates STAT3-dependent intestinal epithelial restitution via increased cell proliferation, decreased apoptosis and upregulation of SERPIN family members. PLoS One. 2014;9:e93498.
Brender C, Nielsen M, Kaltoft K, et al. STAT3-mediated constitutive expression of SOCS-3 in cutaneous T-cell lymphoma. Blood. 2001;97:1056–1062.
Chen J, Xu H, Zou X, et al. Snail recruits Ring1B to mediate transcriptional repression and cell migration in pancreatic cancer cells. Cancer Res. 2014;74:4353–4363.
Shintani Y, Hollingsworth MA, Wheelock MJ, Johnson KR. Collagen I promotes metastasis in pancreatic cancer by activating c-Jun NH(2)-terminal kinase 1 and up-regulating N-cadherin expression. Cancer Res. 2006;66:11745–11753.
Wu HH, Hwang-Verslues WW, Lee WH, et al. Targeting IL-17B-IL-17RB signaling with an anti-IL-17RB antibody blocks pancreatic cancer metastasis by silencing multiple chemokines. J Exp Med. 2015;212:333–349.
Tjomsland V, Niklasson L, Sandström P, et al. The desmoplastic stroma plays an essential role in the accumulation and modulation of infiltrated immune cells in pancreatic adenocarcinoma. Clin Dev Immunol. 2011;2011:212810.
Ueda J, Tanaka M, Ohtsuka T, et al. Surgery for chronic pancreatitis decreases the risk for pancreatic cancer: a multicenter retrospective analysis. Surgery. 2013;153:357–364.
Fukuda A, Wang SC, Morris JP 4th, et al. Stat3 and MMP7 contribute to pancreatic ductal adenocarcinoma initiation and progression. Cancer Cell. 2011;19:441–455.
Lesina M, Kurkowski MU, Ludes K, et al. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell. 2011;19:456–469.
Liu J, Li J, Li H, et al. A comprehensive analysis of candidate genes and pathways in pancreatic cancer. Tumour Biol. 2015;36:1849–1857.
Zhang Y, Yan W, Collins MA, et al. Interleukin-6 is required for pancreatic cancer progression by promoting MAPK signaling activation and oxidative stress resistance. Cancer Res. 2013;73:6359–6374.
Ozdemir BC, Pentcheva-Hoang T, Carstens JL, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25:719–734.
Rhim AD, Oberstein PE, Thomas DH, et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014;25:735–747.
Sherman MH, Yu RT, Engle DD, et al. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell. 2014;159:80–93.
Acknowledgments
This study was supported in part by Grant-in-Aid from the Japan Society for the Promotion of Science (26293171, 26461029, 15H04804), the Mitsui Life Social Welfare Foundation (to A. Masamune), and the Pancreas Research Foundation of Japan (to T. Takikawa).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None declared.
Rights and permissions
About this article
Cite this article
Hamada, S., Masamune, A., Yoshida, N. et al. IL-6/STAT3 Plays a Regulatory Role in the Interaction Between Pancreatic Stellate Cells and Cancer Cells. Dig Dis Sci 61, 1561–1571 (2016). https://doi.org/10.1007/s10620-015-4001-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-015-4001-5