Abstract
Background
Thyroid hormone induces cardiac hypertrophy and preconditions the myocardium against Ischemia/Reperfusion (I/R) injury. Type 2 Angiotensin II receptors (AT2R) are shown to be upregulated in cardiac hypertrophy observed in hyperthyroidism and this receptor has been reported to mediate cardioprotection against ischemic injury.
Methods
The aim of the present study was to evaluate the role of AT2R in the recovery of myocardium after I/R in isolated hearts from T3 treated rats. Male Wistar rats were treated with triiodothyronine (T3; 7 μg/100 g BW/day, i.p.) in the presence or not of a specific AT2R blocker (PD123,319; 10 mg/Kg) for 14 days, while normal rats served as control. After treatment, isolated hearts were perfused in Langendorff mode; after 30 min of stabilization, hearts were subjected to 20 min of zero-flow global ischemia followed by 25 min, 35 min and 45 min of reperfusion.
Results
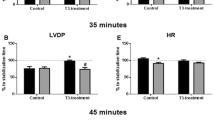
T3 treatment induced cardiac hypertrophy, which was not changed by PD treatment. Post-ischemic recovery of cardiac function was increased in T3-treated hearts after 35 min and 45 min of reperfusion as compared to control and the ischemic contracture was accelerated and intensified. AT2R blockade was able to return the evaluated functional parameters of cardiac performance (LVDP, +dP/dtmáx and −dP/dtmin) to the control condition. Furthermore, AT2R blockade prevented the increase in AMPK expression levels induced by T3, suggesting its possible involvement in this process.
Conclusion
AT2R plays a significant role in T3-induced cardioprotection.






Similar content being viewed by others
References
Dillmann W. Cardiac hypertrophy and thyroid hormone signaling. Heart Fail Rev. 2010;15:125–32.
Fazio S, Palmieri EA, Lombardi G, Biondi B. Effects of thyroid hormone on the cardiovascular system. Recent Prog Horm Res. 2004;59:31–50.
Kahaly GJ, Dillmann WH. Tyroid hormone action in the heart. Endocr Rev. 2005;26:704–28.
Klein I, Ojamaa K. Thyroid hormone: targeting the vascular smooth muscle cell. Circ Res. 2001;88:260–1.
Buser PT, Wikman-Coffelt J, Wu ST, Derugin N, Parmley WW, Higgins CB. Postischaemic recovery of mechanical performance and energy metabolism in the presence of left ventricular hypertrophy. A 31P-MRS study. Circ Res. 1990;66:735–46.
Pantos C, Mourouzis I, Tzeis S, et al. Propranolol diminishes cardiac hypertrophy but does not abolish acceleration of the ischaemic contracture in hyperthyroid hearts. J Cardiovasc Pharmacol. 2000;36:384–9.
Venditti P, Masullo P, Agnisola C, Di Meo S. Effect of vitamin E on the response to ischaemia-reperfusion of Langendorff heart preparations from hyperthyroid rats. Life Sci. 2000;66:697–708.
Pantos C, Malliopoulou V, Mourouzis I, et al. Long-term thyroxine administration protects the heart in a similar pattern as ischaemic preconditioning. Thyroid. 2002;12:325–9.
Pantos C, Malliopoulou V, Paizis I, et al. Thyroid hormone and cardioprotection: study of p38 MAPK and JNKs during ischaemia and at reperfusion in isolated rat heart. Mol Cell Biochem. 2003;242:173–80.
Sirlak M, Yazicoglu L, Inan MB, et al. Oral thyroid hormone pretreatment in left ventricular dysfunction. Eur J Cardiothorac Surg. 2004;26:720–5.
Pantos C, Malliopoulou V, Varonos DD, Cokkinos DV. Thyroid hormone and phenotypes of cardioprotection. Basic Res Cardiol. 2004;99:101–20.
Barreto-Chaves ML, Carrillo-Sepúlveda MA, Carneiro-Ramos MS, Gomes DA, Diniz GP. The crosstalk between thyroid hormones and the renin-angiotensin system. Vascul Pharmacol. 2010;52:166–70.
Hu LW, Benvenutti LA, Liberti EA, Carneiro-Ramos MS, Barreto-Chaves ML. Thyroxine-induced cardiac hypertrophy: influence of adrenergic nervous system versus renin-angiotensin system on myocyte remodeling. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1473–80.
Diniz GP, Carneiro-Ramos MS, Barreto-Chaves ML. Angiotensin type 1 receptor mediates thyroid hormone-induced cardiomyocyte hypertrophy through the Akt/GSK-3beta/mTOR signaling pathway. Basic Res Cardiol. 2009;104:653–67.
Carneiro-Ramos MS, Diniz GP, Nadu AP, et al. Blockage of angiotensin II type 2 receptor prevents thyroxine-mediated cardiac hypertrophy by blocking Akt activation. Basic Res Cardiol. 2010;105:325–35.
De Gasparato M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev. 2000;52:415–72.
Gross V, Obst M, Luft FC. Insights into angiotensin II receptor function through AT2 receptor knockout mice. Acta Physiol Scand. 2004;181:487–94.
Steckelings UM, Widdop RE, Paulis L, Unger T. The angiotensin AT2 receptor in left ventricular hypertrophy. J Hypertens. 2010;28:S50–5.
Yoshiyama M, Kim S, Yamagishi H, et al. Cardioprotective effect of the angiotensin II type 1 receptor antagonist TCV-116 on ischemia-reperfusion injury. Am Heart J. 1994;128:1–6.
Yang BC, Phillips MI, Zhang YC, et al. Critical role of AT1 receptor expression after ischemia/reperfusion in isolated rat hearts: beneficial effect of antisense oligodeoxynucleotides directed at AT1 receptor mRNA. Circ Res. 1998;83:552–9.
Xu Y, Kumar D, Dyck JR, Ford WR, Clanachan AS, Lopaschuk GD, et al. AT(1) and AT(2) receptor expression and blockade after acute ischemia-reperfusion in isolated working rat hearts. Am J Physiol Heart Circ Physiol. 2002;282:H1206–15.
Przyklenk K, Kloner RA. “Cardioprotection” by ACE-inhibitors in acute myocardial ischemia and infarction? Basic Res Cardiol. 1993;88 Suppl 1:139–54.
Pantos C, Paizis I, Mourouzis I, et al. Blockade of angiotensin II type 1 receptor diminishes cardiac hypertrophy, but does not abolish thyroxin-induced preconditioning. Horm Metab Res. 2005;37:500–4.
Langendorff O. Untersuchungen an uberlebenden Saugethierherzen. Pflügers Arch. 1895;61:291–332.
Fallen EL, Elliot WC, Gorlin R. Apparatus for study of ventricular function and metabolism in the isolated perfused rat heart. J Appl Physiol. 1967;22:836–9.
Carrillo-Sepulveda MA, Ceravolo GS, Furstenau CR, et al. Emerging role of angiotensin type 2 receptor (AT2R) /Akt/ NO pathway in vascular smooth muscle cell in the hyperthyroidism. PLoS One. 2013;8:e61982.
Liu YH, Yang XP, Sharov VG, et al. Effects of angiotensin-converting enzyme inhibitors and angiotensin II type 1 receptor antagonists in rats with heart failure. Role of kinins and angiotensin II type 2 receptors. J Clin Invest. 1997;9:1926–35.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54.
Opie LH. Cardioprotection at a distance—remote conditioning takes the stage. Exp Physiol. 2012;97:905.
Pantos C, Malliopoulou V, Mourouzis I, et al. Hyperthyroid hearts display a phenotype of cardioprotection against ischemic stress: a possible involvement of heat shock protein 70. Horm Metab Res. 2006;38:308–13.
Barreto-Chaves ML, de Souza MP, Fürstenau CR. Acute actions of thyroid hormone on blood vessel biochemistry and physiology. Curr Opin Endocrinol Diabetes Obes. 2011;18:300–3.
Diniz GP, Carneiro-Ramos MS, Barreto-Chaves ML. Angiotensin type 1 (AT1) and type 2 (AT2) receptors mediate the increase in TGF-B1 in thyroid hormone-induced cardiac hypertrophy. Pflugers Arch - Eur J Physiol. 2007;454:75–81.
Murphy TJ, Takeuchi K, Alexander RW. Molecular cloning of AT1 angiotensin receptors. Am J Hypertens. 1992;5:236S–42.
Kambayashi Y, Bardhan S, Takahashi K, et al. Molecular cloning of a novel angiotensin II receptor isoform involved in phosphotyrosine phosphatase inhibition. J Biol Chem. 1993;268:24543–6.
Siragy HM, Carey RM. The subtype-2 (AT2) angiotensin receptor regulates renal cyclic guanosine 3′, 5′-monophosphate and AT1 receptor-mediated prostaglandin E2 production in conscious rats. J Clin Invest. 1996;97:1978–82.
Gohlke P, Pees C, Unger T. AT2 receptor stimulation increases aortic cyclic GMP in SHRSP by a kinin-dependent mechanism. Hypertension. 1999;31:349–55.
Batenburg WW, Garrelds IM, Bernasconi CC, et al. Angiotensin II type 2 receptor-mediated vasodilation in human coronary microarteries. Circulation. 2004;109:2296–301.
Tsutsumi Y, Matsubara H, Masaki H, et al. Angiotensin II type 2 receptor overexpression activates the vascular kinin system and causes vasodilation. J Clin Invest. 1999;104:925–35.
Akishita M, Horiuchi M, Yamada H, et al. Inflammation influences vascular remodeling through AT2 receptor expression and signaling. Physiol Genomics. 2000;2:13–20.
Abadir PM, Carey RM, Siragy HM. Angiotensin AT2 receptors directly stimulate renal nitric oxide in bradykinin B2-receptor-null mice. Hypertension. 2003;42:600–4.
Hermann R, Marina Prendes MG, Torresin ME, Vélez D, Savino EA, Varela A. Effects of the AMP-activated protein kinase inhibitor compound C on the postconditioned rat heart. J Physiol Sci. 2012;62:333–41.
Thuc LC, Teshima Y, Takahashi N, et al. Cardioprotective effects of pravastatin against lethal ventricular arrhythmias induced by reperfusion in the rat heart. Circ J. 2011;75:1601–8.
Oishi Y, Ozono R, Yano Y, et al. Cardioprotective role of AT2 receptor in postinfarction left ventricular remodeling. Hypertension. 2003;41:814–8.
Calvert JW, Gundewar S, Jha S, et al. Acute metformin therapy confers cardioprotection against myocardial infarction via AMPK-eNOS-mediated signaling. Diabetes. 2008;57:696–705.
Gundewar S, Calvert JW, Jha S, et al. Activation of AMP-activated protein kinase by metformin improves left ventricular function and survival in heart failure. Circ Res. 2009;104:403–11.
Olson AK, Bouchard B, Ning XH, Isern M, Rosiers CD, Portman MA. Triodothironine increases myocardial function and pyruvate entry into the citric acid cycle after reperfusion in a model of infant cardiopulmonary bypass. Am J Physiol Heart Circ Physiol. 2012;302:H1086–93.
Acknowledgments
FM Tavares and IB da Silva contributed equally to this work.
We thank Dr. DaltonVassalo from Department of Physiology, University of Espirito Santo, Brazil, for the contribution with numerous suggestions during this study.
This study received financial support in the form of grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, Foundation for the Support of Research in the State of Sao Paulo) and ConselhoNacional de DesenvolvimentoCientífico e Tecnológico (CNPq, National Council for Scientific and Technological Development).
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Felix Meira Tavares and Ivson Bezerra da Silva contributed equally to this work.
Rights and permissions
About this article
Cite this article
Tavares, F.M., da Silva, I.B., Gomes, D.A. et al. Angiotensin II Type 2 Receptor (AT2R) is Associated with Increased Tolerance of the Hyperthyroid Heart to Ischemia-Reperfusion. Cardiovasc Drugs Ther 27, 393–402 (2013). https://doi.org/10.1007/s10557-013-6473-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-013-6473-x