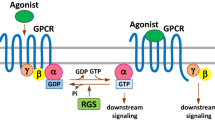
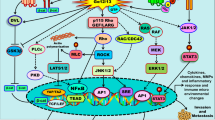
Abstract
G protein-coupled receptors (GPCRs) comprise the main signal-transmitting components in the cell membrane. Over the past several years, biochemical and structural analyses have immensely enhanced our knowledge of GPCR involvement in health and disease states. The present review focuses on GPCRs that are cancer drivers, involved in tumor growth and development. Our aim is to highlight the involvement of stabilized β-catenin molecular machinery with a specific array of GPCRs. We discuss recent advances in understanding the molecular path leading to β-catenin nuclear localization and transcriptional activity and their implications for future cancer therapy research.


Similar content being viewed by others
Change history
20 December 2017
The original version of this article unfortunately contained a mistake. The family name of Beatrice Uziely was mistakenly spelled as Uzieky. The correct name is now presented above.
References
Bjarnadottir, T. K., Gloriam, D. E., Hellstrand, S. H., Kristiansson, H., Fredriksson, R., & Schioth, H. B. (2006). Comprehensive repertoire and phylogenetic analysis of the G protein-coupled receptors in human and mouse. Genomics, 88(3), 263–273.
Bockaert, J., & Pin, J. P. (1999). Molecular tinkering of G protein-coupled receptors: an evolutionary success. The EMBO Journal, 18(7), 1723–1729.
Feigin, M. E. (2013). Harnessing the genome for characterization of G-protein coupled receptors in cancer pathogenesis. The FEBS Journal, 280(19), 4729–4738.
Hollenberg, M. D., Mihara, K., Polley, D., Suen, J. Y., Han, A., Fairlie, D. P., et al. (2014). Biased signalling and proteinase-activated receptors (PARs): targeting inflammatory disease. British Journal of Pharmacology, 171(5), 1180–1194.
Kenakin, T. (2012). The potential for selective pharmacological therapies through biased receptor signaling. BMC Pharmacology & Toxicology, 13, 3.
Miao, Y., & McCammon, J. A. (2016). G-protein coupled receptors: advances in simulation and drug discovery. Current Opinion in Structural Biology, 41, 83–89.
Wisler, J. W., Xiao, K., Thomsen, A. R., & Lefkowitz, R. J. (2014). Recent developments in biased agonism. Current Opinion in Cell Biology, 27, 18–24.
Premont, R. T., & Gainetdinov, R. R. (2007). Physiological roles of G protein-coupled receptor kinases and arrestins. Annual Review of Physiology, 69, 511–534.
Clevers, H. (2006). Wnt/beta-catenin signaling in development and disease. Cell, 127(3), 469–480.
Nusse, R. (2005). Wnt signaling in disease and in development. Cell Research, 15(1), 28–32.
Liu, T., DeCostanzo, A. J., Liu, X., Wang, H., Hallagan, S., Moon, R. T., et al. (2001). G protein signaling from activated rat frizzled-1 to the beta-catenin-Lef-Tcf pathway. Science, 292(5522), 1718–1722.
Wang, H. Y., & Malbon, C. C. (2004). Wnt-frizzled signaling to G-protein-coupled effectors. Cellular and Molecular Life Sciences: CMLS, 61(1), 69–75.
Jilka, R. L. (2007). Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone, 40(6), 1434–1446.
Potts, J. T., & Gardella, T. J. (2007). Progress, paradox, and potential: parathyroid hormone research over five decades. Annals of the New York Academy of Sciences, 1117, 196–208.
Honore, P., Luger, N. M., Sabino, M. A., Schwei, M. J., Rogers, S. D., Mach, D. B., et al. (2000). Osteoprotegerin blocks bone cancer-induced skeletal destruction, skeletal pain and pain-related neurochemical reorganization of the spinal cord. Nature Medicine, 6(5), 521–528.
Southby, J., Kissin, M. W., Danks, J. A., Hayman, J. A., Moseley, J. M., Henderson, M. A., et al. (1990). Immunohistochemical localization of parathyroid hormone-related protein in human breast cancer. Cancer Research, 50(23), 7710–7716.
McCauley, L. K., & Martin, T. J. (2012). Twenty-five years of PTHrP progress: from cancer hormone to multifunctional cytokine. Journal of Bone and Mineral Research: the Official Journal of the American Society for Bone and Mineral Research, 27(6), 1231–1239.
Allison, D. C., Carney, S. C., Ahlmann, E. R., Hendifar, A., Chawla, S., Fedenko, A., et al. (2012). A meta-analysis of osteosarcoma outcomes in the modern medical era. Sarcoma, 2012, 704872.
Fearon, K., Arends, J., & Baracos, V. (2013). Understanding the mechanisms and treatment options in cancer cachexia. Nature Reviews. Clinical Oncology, 10(2), 90–99.
Fearon, K. C., Glass, D. J., & Guttridge, D. C. (2012). Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metabolism, 16(2), 153–166.
Kir, S., White, J. P., Kleiner, S., Kazak, L., Cohen, P., Baracos, V. E., et al. (2014). Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Nature, 513(7516), 100–104.
Ovesen, L., Allingstrup, L., Hannibal, J., Mortensen, E. L., & Hansen, O. P. (1993). Effect of dietary counseling on food intake, body weight, response rate, survival, and quality of life in cancer patients undergoing chemotherapy: a prospective, randomized study. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology, 11(10), 2043–2049.
Tisdale, M. J. (2009). Mechanisms of cancer cachexia. Physiological Reviews, 89(2), 381–410. https://doi.org/10.1152/physrev.00016.2008.
Barnes, M. R., Duckworth, D. M., & Beeley, L. J. (1998). Frizzled proteins constitute a novel family of G protein-coupled receptors, most closely related to the secretin family. Trends in Pharmacological Sciences, 19(10), 399–400.
Romero, G., Sneddon, W. B., Yang, Y., Wheeler, D., Blair, H. C., & Friedman, P. A. (2010). Parathyroid hormone receptor directly interacts with dishevelled to regulate beta-catenin signaling and osteoclastogenesis. The Journal of Biological Chemistry, 285(19), 14756–14763.
Boyce, B. F., Xing, L., & Chen, D. (2005). Osteoprotegerin, the bone protector, is a surprising target for beta-catenin signaling. Cell Metabolism, 2(6), 344–345.
Goldring, S. R., & Goldring, M. B. (2007). Eating bone or adding it: the Wnt pathway decides. Nature Medicine, 13(2), 133–134.
Holmen, S. L., Zylstra, C. R., Mukherjee, A., Sigler, R. E., Faugere, M. C., Bouxsein, M. L., et al. (2005). Essential role of beta-catenin in postnatal bone acquisition. The Journal of Biological Chemistry, 280(22), 21162–21168.
Yao, W., Cheng, Z., Shahnazari, M., Dai, W., Johnson, M. L., & Lane, N. E. (2010). Overexpression of secreted frizzled-related protein 1 inhibits bone formation and attenuates parathyroid hormone bone anabolic effects. Journal of Bone and Mineral Research: the Official Journal of the American Society for Bone and Mineral Research, 25(2), 190–199.
Diarra, D., Stolina, M., Polzer, K., Zwerina, J., Ominsky, M. S., Dwyer, D., et al. (2007). Dickkopf-1 is a master regulator of joint remodeling. Nature Medicine, 13(2), 156–163.
Wan, M., Yang, C., Li, J., Wu, X., Yuan, H., Ma, H., et al. (2008). Parathyroid hormone signaling through low-density lipoprotein-related protein 6. Genes & Development, 22(21), 2968–2979.
Torrance, C. J., Jackson, P. E., Montgomery, E., Kinzler, K. W., Vogelstein, B., Wissner, A., et al. (2000). Combinatorial chemoprevention of intestinal neoplasia. Nature Medicine, 6(9), 1024–1028.
Subbaramaiah, K., & Dannenberg, A. J. (2003). Cyclooxygenase 2: a molecular target for cancer prevention and treatment. Trends in Pharmacological Sciences, 24(2), 96–102. https://doi.org/10.1016/S0165-6147(02)00043-3.
Vane, J. R., & Botting, R. M. (1998). Mechanism of action of nonsteroidal anti-inflammatory drugs. The American Journal of Medicine, 104(3A), 2S–8S discussion 21S-22S.
Castellone, M. D., Teramoto, H., Williams, B. O., Druey, K. M., & Gutkind, J. S. (2005). Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-beta-catenin signaling axis. Science, 310(5753), 1504–1510.
Hull, M. A., Ko, S. C., & Hawcroft, G. (2004). Prostaglandin EP receptors: targets for treatment and prevention of colorectal cancer? Molecular Cancer Therapeutics, 3(8), 1031–1039.
Backlund, M. G., Mann, J. R., & Dubois, R. N. (2005). Mechanisms for the prevention of gastrointestinal cancer: the role of prostaglandin E2. Oncology, 69(Suppl 1), 28–32.
Wang, D., Wang, H., Brown, J., Daikoku, T., Ning, W., Shi, Q., et al. (2006). CXCL1 induced by prostaglandin E2 promotes angiogenesis in colorectal cancer. The Journal of Experimental Medicine, 203(4), 941–951.
Majumder, M., Xin, X., Liu, L., Tutunea-Fatan, E., Rodriguez-Torres, M., Vincent, K., et al. (2016). COX-2 induces breast cancer stem cells via EP4/PI3K/AKT/NOTCH/WNT Axis. Stem Cells, 34(9), 2290–2305.
Du, M., Shi, F., Zhang, H., Xia, S., Zhang, M., Ma, J., et al. (2015). Prostaglandin E2 promotes human cholangiocarcinoma cell proliferation, migration and invasion through the upregulation of beta-catenin expression via EP3-4 receptor. Oncology Reports, 34(2), 715–726.
Vaid, M., Singh, T., Prasad, R., Kappes, J. C., & Katiyar, S. K. (2015). Therapeutic intervention of proanthocyanidins on the migration capacity of melanoma cells is mediated through PGE2 receptors and beta-catenin signaling molecules. American Journal of Cancer Research, 5(11), 3325–3338.
Auersperg, N. (2011). The origin of ovarian carcinomas: a unifying hypothesis. International Journal of Gynecological Pathology: Official Journal of the International Society of Gynecological Pathologists, 30(1), 12–21.
Kim, J., Coffey, D. M., Creighton, C. J., Yu, Z., Hawkins, S. M., & Matzuk, M. M. (2012). High-grade serous ovarian cancer arises from fallopian tube in a mouse model. Proceedings of the National Academy of Sciences of the United States of America, 109(10), 3921–3926.
Kurman, R. J., & Shih Ie, M. (2010). The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. The American Journal of Surgical Pathology, 34(3), 433–443.
Kurman, R. J., & Shih Ie, M. (2011). Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer—shifting the paradigm. Human Pathology, 42(7), 918–931. https://doi.org/10.1016/j.humpath.2011.03.003.
Lee, Y., Miron, A., Drapkin, R., Nucci, M. R., Medeiros, F., Saleemuddin, A., et al. (2007). A candidate precursor to serous carcinoma that originates in the distal fallopian tube. The Journal of Pathology, 211(1), 26–35.
Shih Ie, M., & Kurman, R. J. (2004). Ovarian tumorigenesis: a proposed model based on morphological and molecular genetic analysis. The American Journal of Pathology, 164(5), 1511–1518.
Auersperg, N., Edelson, M. I., Mok, S. C., Johnson, S. W., & Hamilton, T. C. (1998). The biology of ovarian cancer. Seminars in Oncology, 25(3), 281–304.
Lengyel, E. (2010). Ovarian cancer development and metastasis. The American Journal of Pathology, 177(3), 1053–1064.
Mills, G. B., May, C., Hill, M., Campbell, S., Shaw, P., & Marks, A. (1990). Ascitic fluid from human ovarian cancer patients contains growth factors necessary for intraperitoneal growth of human ovarian adenocarcinoma cells. The Journal of Clinical Investigation, 86(3), 851–855.
Xu, Y., Gaudette, D. C., Boynton, J. D., Frankel, A., Fang, X. J., Sharma, A., et al. (1995). Characterization of an ovarian cancer activating factor in ascites from ovarian cancer patients. Clinical Cancer Research: an Official Journal of the American Association for Cancer Research, 1(10), 1223–1232.
Xu, Y., Shen, Z., Wiper, D. W., Wu, M., Morton, R. E., Elson, P., et al. (1998). Lysophosphatidic acid as a potential biomarker for ovarian and other gynecologic cancers. JAMA, 280(8), 719–723.
Hecht, J. H., Weiner, J. A., Post, S. R., & Chun, J. (1996). Ventricular zone gene-1 (vzg-1) encodes a lysophosphatidic acid receptor expressed in neurogenic regions of the developing cerebral cortex. The Journal of Cell Biology, 135(4), 1071–1083.
Yang, M., Zhong, W. W., Srivastava, N., Slavin, A., Yang, J., Hoey, T., et al. (2005). G protein-coupled lysophosphatidic acid receptors stimulate proliferation of colon cancer cells through the beta-catenin pathway. Proceedings of the National Academy of Sciences of the United States of America, 102(17), 6027–6032.
Rosanò, L., Spinella, F., & Bagnato, A. (2013). Endothelin 1 in cancer: biological implications and therapeutic opportunities. Nature Reviews. Cancer, 13(9), 637–651. https://doi.org/10.1038/nrc3546.
Ranjan, R., Dwivedi, H., Baidya, M., Kumar, M., & Shukla, A. K. (2017). Novel structural insights into GPCR-beta-arrestin interaction and signaling. Trends in Cell Biology, S0962-8924(17), 30087–30089.
Cahill 3rd, T. J., Thomsen, A. R., Tarrasch, J. T., Plouffe, B., Nguyen, A. H., Yang, F., et al. (2017). Distinct conformations of GPCR-beta-arrestin complexes mediate desensitization, signaling, and endocytosis. Proceedings of the National Academy of Sciences of the United States of America, 114(10), 2562–2567.
Jean-Charles, P. Y., Kaur, S., & Shenoy, S. K. (2017). G protein-coupled receptor signaling through beta-arrestin-dependent mechanisms. Journal of Cardiovascular Pharmacology, 70(3), 142–158.
Hinsley, E. E., Hunt, S., Hunter, K. D., Whawell, S. A., & Lambert, D. W. (2012). Endothelin-1 stimulates motility of head and neck squamous carcinoma cells by promoting stromal-epithelial interactions. International Journal of Cancer, 130(1), 40–47.
Kim, T. H., Xiong, H., Zhang, Z., & Ren, B. (2005). Beta-catenin activates the growth factor endothelin-1 in colon cancer cells. Oncogene, 24(4), 597–604.
Rosanò, L., Cianfrocca, R., Masi, S., Spinella, F., Di Castro, V., Biroccio, A., et al. (2009). Beta-arrestin links endothelin A receptor to beta-catenin signaling to induce ovarian cancer cell invasion and metastasis. Proceedings of the National Academy of Sciences of the United States of America, 106(8), 2806–2811.
Rosanò, L., Cianfrocca, R., Tocci, P., Spinella, F., Di Castro, V., Spadaro, F., et al. (2013). Beta-arrestin-1 is a nuclear transcriptional regulator of endothelin-1-induced beta-catenin signaling. Oncogene, 32(42), 5066–5077.
Spinella, F., Caprara, V., Di Castro, V., Rosano, L., Cianfrocca, R., Natali, P. G., et al. (2013). Endothelin-1 induces the transactivation of vascular endothelial growth factor receptor-3 and modulates cell migration and vasculogenic mimicry in melanoma cells. Journal of Molecular Medicine, 91(3), 395–405.
Sun, P., Xiong, H., Kim, T. H., Ren, B., & Zhang, Z. (2006). Positive inter-regulation between beta-catenin/T cell factor-4 signaling and endothelin-1 signaling potentiates proliferation and survival of prostate cancer cells. Molecular Pharmacology, 69(2), 520–531.
de Lau, W., Barker, N., Low, T. Y., Koo, B. K., Li, V. S., Teunissen, H., et al. (2011). Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature, 476(7360), 293–297.
Carmon, K. S., Gong, X., Lin, Q., Thomas, A., & Liu, Q. (2011). R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/beta-catenin signaling. Proceedings of the National Academy of Sciences of the United States of America, 108(28), 11452–11457.
Hao, H. X., Xie, Y., Zhang, Y., Charlat, O., Oster, E., Avello, M., et al. (2012). ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature, 485(7397), 195–200.
Koo, B. K., Spit, M., Jordens, I., Low, T. Y., Stange, D. E., van de Wetering, M., et al. (2012). Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nature, 488(7413), 665–669.
Barker, N., Ridgway, R. A., van Es, J. H., van de Wetering, M., Begthel, H., van den Born, M., et al. (2009). Crypt stem cells as the cells-of-origin of intestinal cancer. Nature, 457(7229), 608–611.
Barker, N., Tan, S., & Clevers, H. (2013). Lgr proteins in epithelial stem cell biology. Development, 140(12), 2484–2494.
Munoz, J., Stange, D. E., Schepers, A. G., van de Wetering, M., Koo, B. K., Itzkovitz, S., et al. (2012). The Lgr5 intestinal stem cell signature: robust expression of proposed quiescent '+4' cell markers. The EMBO Journal, 31(14), 3079–3091.
van der Flier, L. G., van Gijn, M. E., Hatzis, P., Kujala, P., Haegebarth, A., Stange, D. E., et al. (2009). Transcription factor achaete scute-like 2 controls intestinal stem cell fate. Cell, 136(5), 903–912.
Ramalingam, S., Daughtridge, G. W., Johnston, M. J., Gracz, A. D., & Magness, S. T. (2012). Distinct levels of Sox9 expression mark colon epithelial stem cells that form colonoids in culture. American Journal of Physiology. Gastrointestinal and Liver Physiology, 302(1), G10–G20.
Furuyama, K., Kawaguchi, Y., Akiyama, H., Horiguchi, M., Kodama, S., Kuhara, T., et al. (2011). Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nature Genetics, 43(1), 34–41.
Clevers, H. (2013). The intestinal crypt, a prototype stem cell compartment. Cell, 154(2), 274–284.
Reya, T., & Clevers, H. (2005). Wnt signalling in stem cells and cancer. Nature, 434(7035), 843–850.
Logan, C. Y., & Nusse, R. (2004). The Wnt signaling pathway in development and disease. Annual Review of Cell and Developmental Biology, 20, 781–810.
Marikawa, Y. (2006). Wnt/beta-catenin signaling and body plan formation in mouse embryos. Seminars in Cell & Developmental Biology, 17(2), 175–184.
Harland, R., & Gerhart, J. (1997). Formation and function of Spemann’s organizer. Annual Review of Cell and Developmental Biology, 13, 611–667.
Korinek, V., Barker, N., Morin, P. J., van Wichen, D., de Weger, R., Kinzler, K. W., et al. (1997). Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC-/- colon carcinoma. Science, 275(5307), 1784–1787.
Valenta, T., Hausmann, G., & Basler, K. (2012). The many faces and functions of beta-catenin. The EMBO Journal, 31(12), 2714–2736.
Major, M. B., Roberts, B. S., Berndt, J. D., Marine, S., Anastas, J., Chung, N., et al. (2008). New regulators of Wnt/beta-catenin signaling revealed by integrative molecular screening. Science Signaling, 1(45), ra12.
Regard, J. B., Cherman, N., Palmer, D., Kuznetsov, S. A., Celi, F. S., Guettier, J. M., et al. (2011). Wnt/beta-catenin signaling is differentially regulated by Galpha proteins and contributes to fibrous dysplasia. Proceedings of the National Academy of Sciences of the United States of America, 108(50), 20101–20106.
Katanaev, V. L., Ponzielli, R., Semeriva, M., & Tomlinson, A. (2005). Trimeric G protein-dependent frizzled signaling in Drosophila. Cell, 120(1), 111–122.
Mo, J. S., Yu, F. X., Gong, R., Brown, J. H., & Guan, K. L. (2012). Regulation of the Hippo-YAP pathway by protease-activated receptors (PARs). Genes & Development, 26(19), 2138–2143.
Pan, D. (2010). The hippo signaling pathway in development and cancer. Developmental Cell, 19(4), 491–505.
Slusarski, D. C., Corces, V. G., & Moon, R. T. (1997). Interaction of Wnt and a Frizzled homologue triggers G-protein-linked phosphatidylinositol signalling. Nature, 390(6658), 410–413.
Feng, X., Degese, M. S., Iglesias-Bartolome, R., Vaque, J. P., Molinolo, A. A., Rodrigues, M., et al. (2014). Hippo-independent activation of YAP by the GNAQ uveal melanoma oncogene through a trio-regulated rho GTPase signaling circuitry. Cancer Cell, 25(6), 831–845.
Park, H. W., Kim, Y. C., Yu, B., Moroishi, T., Mo, J. S., Plouffe, S. W., et al. (2015). Alternative Wnt signaling activates YAP/TAZ. Cell, 162(4), 780–794.
Meng, Z., Moroishi, T., & Guan, K. L. (2016). Mechanisms of Hippo pathway regulation. Genes & Development, 30(1), 1–17.
Yu, F. X., Zhao, B., Panupinthu, N., Jewell, J. L., Lian, I., Wang, L. H., et al. (2012). Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell, 150(4), 780–791.
Burger, M. M. (1970). Proteolytic enzymes initiating cell division and escape from contact inhibition of growth. Nature, 227(5254), 170–171.
Carney, D. H., & Cunningham, D. D. (1977). Initiation of check cell division by trypsin action at the cell surface. Nature, 268(5621), 602–606.
Chen, L. B., & Buchanan, J. M. (1975). Mitogenic activity of blood components. I. Thrombin and prothrombin. Proceedings of the National Academy of Sciences of the United States of America, 72(1), 131–135.
Rasmussen, U. B., Vouret-Craviari, V., Jallat, S., Schlesinger, Y., Pages, G., Pavirani, A., et al. (1991). cDNA cloning and expression of a hamster alpha-thrombin receptor coupled to Ca2+ mobilization. FEBS Letters, 288(1–2), 123–128.
Vu, T. K., Hung, D. T., Wheaton, V. I., & Coughlin, S. R. (1991). Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell, 64(6), 1057–1068.
Coughlin, S. R. (1994). Protease-activated receptors start a family. Proceedings of the National Academy of Sciences of the United States of America, 91(20), 9200–9202.
Nystedt, S., Emilsson, K., Wahlestedt, C., & Sundelin, J. (1994). Molecular cloning of a potential proteinase activated receptor. Proceedings of the National Academy of Sciences of the United States of America, 91(20), 9208–9212.
O'Brien, P. J., Prevost, N., Molino, M., Hollinger, M. K., Woolkalis, M. J., Woulfe, D. S., et al. (2000). Thrombin responses in human endothelial cells. Contributions from receptors other than PAR1 include the transactivation of PAR2 by thrombin-cleaved PAR1. The Journal of Biological Chemistry, 275(18), 13502–13509.
Jaber, M., Maoz, M., Kancharla, A., Agranovich, D., Peretz, T., Grisaru-Granovsky, S., et al. (2014). Protease-activated-receptor-2 affects protease-activated-receptor-1-driven breast cancer. Cellular and Molecular Life Sciences: CMLS, 71(13), 2517–2533.
Sevigny, L. M., Austin, K. M., Zhang, P., Kasuda, S., Koukos, G., Sharifi, S., et al. (2011). Protease-activated receptor-2 modulates protease-activated receptor-1-driven neointimal hyperplasia. Arteriosclerosis, Thrombosis, and Vascular Biology, 31(12), e100–e106.
McLaughlin, J. N., Patterson, M. M., & Malik, A. B. (2007). Protease-activated receptor-3 (PAR3) regulates PAR1 signaling by receptor dimerization. Proceedings of the National Academy of Sciences of the United States of America, 104(13), 5662–5667.
Leger, A. J., Jacques, S. L., Badar, J., Kaneider, N. C., Derian, C. K., Andrade-Gordon, P., et al. (2006). Blocking the protease-activated receptor 1-4 heterodimer in platelet-mediated thrombosis. Circulation, 113(9), 1244–1254.
Blackhart, B. D., Emilsson, K., Nguyen, D., Teng, W., Martelli, A. J., Nystedt, S., et al. (1996). Ligand cross-reactivity within the protease-activated receptor family. The Journal of Biological Chemistry, 271(28), 16466–16471.
Ishihara, H., Connolly, A. J., Zeng, D., Kahn, M. L., Zheng, Y. W., Timmons, C., et al. (1997). Protease-activated receptor 3 is a second thrombin receptor in humans. Nature, 386(6624), 502–506.
Kahn, M. L., Zheng, Y. W., Huang, W., Bigornia, V., Zeng, D., Moff, S., et al. (1998). A dual thrombin receptor system for platelet activation. Nature, 394(6694), 690–694.
Xu, W. F., Andersen, H., Whitmore, T. E., Presnell, S. R., Yee, D. P., Ching, A., et al. (1998). Cloning and characterization of human protease-activated receptor 4. Proceedings of the National Academy of Sciences of the United States of America, 95(12), 6642–6646.
Even-Ram, S., Uziely, B., Cohen, P., Grisaru-Granovsky, S., Maoz, M., Ginzburg, Y., et al. (1998). Thrombin receptor overexpression in malignant and physiological invasion processes. Nature Medicine, 4(8), 909–914.
Nag, J. K., Kancharla, A., Maoz, M., Turm, H., Agranovich, D., Gupta, C. L., et al. (2017). Low-density lipoprotein receptor-related protein 6 is a novel coreceptor of protease-activated receptor-2 in the dynamics of cancer-associated beta-catenin stabilization. Oncotarget, 8(24), 38650–38667.
Kancharla, A., Maoz, M., Jaber, M., Agranovich, D., Peretz, T., Grisaru-Granovsky, S., et al. (2015). PH motifs in PAR1&2 endow breast cancer growth. Nature Communications, 6, 8853–8865.
Yin, Y. J., Katz, V., Salah, Z., Maoz, M., Cohen, I., Uziely, B., et al. (2006). Mammary gland tissue targeted overexpression of human protease-activated receptor 1 reveals a novel link to beta-catenin stabilization. Cancer Research, 66(10), 5224–5233.
Author information
Authors and Affiliations
Corresponding author
Additional information
The original version of this article was revised: The family name of Beatrice Uziely was mistakenly spelled as Uzieky. The correct name is now presented above.
A correction to this article is available online at https://doi.org/10.1007/s10555-017-9721-x.
Rights and permissions
About this article
Cite this article
Nag, J.K., Rudina, T., Maoz, M. et al. Cancer driver G-protein coupled receptor (GPCR) induced β-catenin nuclear localization: the transcriptional junction. Cancer Metastasis Rev 37, 147–157 (2018). https://doi.org/10.1007/s10555-017-9711-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-017-9711-z