Abstract
Cognitive or executive control is a critical mental ability, an important marker of mental illness, and among the most heritable of neurocognitive traits. Two candidate genes, catechol-O-methyltransferase (COMT) and DRD4, which both have a roles in the regulation of cortical dopamine, have been consistently associated with cognitive control. Here, we predicted that individuals with the COMT Met/Met allele would show improved response execution and inhibition as indexed by event-related potentials in a Go/NoGo task, while individuals with the DRD4 7-repeat allele would show impaired brain activity. We used independent component analysis (ICA) to separate brain source processes contributing to high-density EEG scalp signals recorded during the task. As expected, individuals with the DRD4 7-repeat polymorphism had reduced parietal P3 source and scalp responses to response (Go) compared to those without the 7-repeat. Contrary to our expectation, the COMT homozygous Met allele was associated with a smaller frontal P3 source and scalp response to response-inhibition (NoGo) stimuli, suggesting that while more dopamine in frontal cortical areas has advantages in some tasks, it may also compromise response inhibition function. An interaction effect emerged for P3 source responses to Go stimuli. These were reduced in those with both the 7-repeat DRD4 allele and either the COMT Val/Val or the Met/Met homozygous polymorphisms but not in those with the heterozygous Val/Met polymorphism. This epistatic interaction between DRD4 and COMT replicates findings that too little or too much dopamine impairs cognitive control. The anatomic and functional separated maximally independent cortical EEG sources proved more informative than scalp channel measures for genetic studies of brain function and thus better elucidate the complex mechanisms in psychiatric illness.
Similar content being viewed by others
Introduction
The global health burden of psychiatric illness (Whiteford et al. 2013) and its serious consequences for the individual and society (Organization 2014) have encouraged recent efforts to improve understanding of the etiology and pathophysiology of mental disorders. More precise phenotyping of disorders could in turn result in better diagnostics and therapeutics (Insel 2014). Despite the high heritability estimates for most major psychiatric disorders, including attention deficit hyperactivity disorder (ADHD), autism spectrum disorders (ASD), bipolar disorder and schizophrenia (Committee 2009), there are still few genetic markers that reliably associate with these disorders and limited significant genome-wide associations have been documented to date (Sullivan 2011). One possible reason for this is that psychiatric disorders are often heterogeneous at the symptom level and thus uncertainties about phenotype definition may have impeded the discovery of associated genetic risk factors (McLoughlin et al. 2013). A current goal for research in psychiatry is to close the gap in understanding between the symptoms and causes of psychopathology, and move beyond subjective and variable clinical diagnoses to classify disorders based on identifiable neural circuits, and further to link activity in these circuits to the cellular and genetic levels (Insel and Cuthbert 2009).
Within psychiatry, the concept of an endophenotype refers to a heritable quantitative trait, either cognitive or neurophysiological, that is more directly related to dysfunction in neural systems than diagnosis, and which therefore facilitates the identification of genetic variants associated with psychopathology (Gottesman and Gould 2003). Ideally an endophenotype should be heritable, associated with disorder diagnosis and be present in unaffected family members (Hawi et al. 2015). Brain dynamic measures known to relate to the pathophysiology of psychiatric illness can direct search for disorder endophenotypes. One strategy for identifying such measures is to examine cognitive and neural dysfunction closely related to core behavioural symptoms.
Cognitive or executive control, the capacity to flexibly direct and allocate resources to a goal by selecting and integrating relevant contextual information, is critical for higher mental abilities (Blasi et al. 2005). Cognitive control is among the most heritable of neurocognitive traits (Anokhin et al. 2004; Friedman et al. 2008; Macare et al. 2014) and deficits in cognitive control are consistently associated with multiple psychiatric illnesses (Laucht et al. 1997a; Millan et al. 2012; Nieoullon 2002; Royall et al. 2002). Thus there has been a focus on genetic approaches to uncover the biological underpinnings of these measures. Converging evidence from pharmacological, neuroimaging and animal studies indicates that dopamine is critical for the efficiency of cognitive control (for a review, see Cools 2008). Specifically, numerous studies suggest that too little or too much dopamine in the cortex, in particular in the prefrontal cortex (PFC), can be disruptive, impacting both brain dynamics and performance on tasks requiring cognitive control (Bruggemann et al. 2013; Laurens et al. 2010; Luu et al. 2004).
Recent evidence indicates that catechol-O-methyltransferase (COMT), which has a crucial role in the regulation of dopamine in the PFC, accounts for some individual variability on tests of executive or cognitive control (Dickinson and Elvevåg 2009; Goldberg and Weinberger 2004). The common polymorphism (Val158Met) predicts activity of COMT; homozygosity for the COMT Met allele is associated with a 35–50% decrease in enzymatic activity and dopamine catabolism relative to Val homozygosity, ultimately resulting in increased availability of dopamine in PFC (Biederman et al. 2008; DeYoung et al. 2010; Dickinson and Elvevåg 2009; Lachman et al. 1996; O’Sullivan et al. 2009). Individuals with the homozygous Met allele (and concomitant PFC dopamine enhancement) show improved cognitive control, working memory, and general intelligence as indexed by both performance, and correlated event-related potential (ERP) and functional magnetic resonance imaging (fMRI) measures (Bertolino et al. 2004; Blasi et al. 2005; Cahoy et al. 2008; Diamond et al. 2014; Eroglu and Barres 2010; Freeman and Rowitch 2013; Joober et al. 2002; Wong and Van Tol 2003). However, other studies using cognitive performance, ERP and transcranial direct-current stimulation (tDCS) measures (Plewnia et al. 2013; Stefanis et al. 2004; Taerk et al. 2004; Tsai et al. 2003) have reported no association between COMT and cognitive control, or even that homozygous Met carriers exhibit abnormal cognitive control activity (Gallinat et al. 2003; Kramer et al. 2007).
Another polymorphism important for understanding genetic effects on executive control is the 7-repeat polymorphism of the DRD4 gene, which decreases dopamine transmission at the D4 receptor (Bellgrove et al. 2005; Johnson et al. 2008; Kiphardt 1974). D4 receptor function may play a role in the etiology of both personality traits and psychiatric illness (Ptacek et al. 2011) with a particular relation to ADHD—an association with the 7-repeat allele is the strongest and most consistently replicated molecular genetic finding in the disorder (Banaschewski et al. 2010; Faraone et al. 2001; Li et al. 2006). Participants carrying the 7-repeat allele also have been reported to show poorer performance on tasks of attentional and executive functions and to have smaller attention-related ERP peaks in such tasks (Albrecht et al. 2014; Vogel et al. 2006). However, findings have again been inconsistent. Some studies have reported that the 7-repeat allele is associated with better performance on executive function tasks (Johnson et al. 2008; Swanson et al. 2000), while other studies have found no such differences (Barkley et al. 2006; Konrad et al. 2010).
Disparities between the results of studies investigating the role of COMT and DRD4 in cognitive control may relate to the use of psychiatric populations (Bertolino et al. 2004; Ehlis et al. 2007; Eroglu and Barres 2010; Stefanis et al. 2004; Taerk et al. 2004) or to the effects of other interacting genes (Wishart et al. 2011). Given that the level of dopamine in the cortex has specific effects on cognitive performance (either too much or too little can lead to impairments) (Bruggemann et al. 2013; Laurens et al. 2010; Luu et al. 2004) it is likely that the epistatic interaction between COMT and DRD4 has an impact on dopaminergic influences on cognitive control. Indeed, one recent study showed no impact of the single genes on an ERP-derived measure of response control (the “NoGo anteriorisation” or NGA) (Fallgatter et al. 1997), whereas there was a strong interaction between COMT and DRD4 on NGA amplitude and also on reaction time variability (Takahashi et al. 2011). This interaction effect may not extend to reward-related processing as measured by ERP markers to feedback on gains and losses (Cavanagh et al. 2012).
Another possible reason for not replicating COMT and DRD4 effects in cognitive control tasks is the relative inffectiveness of the measures used to characterise the underlying neurophysiology of the complex cognitive sub-processes (Cools 2008). Cognitive control has a multifactorial nature. Cognitive electrophysiology as well as functional imaging studies using fMRI have demonstrated that it is associated with activity in a network of brain regions, in particular the basal ganglia, prefrontal, and parietal attentional systems including medial frontal and dorsolateral prefrontal cortex, anterior cingulate cortex (ACC), and precuneus (Ogg et al. 2008; Yoon et al. 2008). Indeed there is evidence that the effects of COMT and DRD4 may contribute to anterior-posterior functional connectivity (Jaspar et al. 2014; Liu et al. 2010; Tian et al. 2013) and thus extend beyond the PFC to areas including the dorsal anterior cingulate (Blasi et al. 2005).
However, it should be noted that the associations previously found between COMT, DRD4, and cognitive ERP measures are not exclusive, and certainly do not suggest a one-to-one relation between dopamine-related gene functions and ERP component measures of cognitive control subprocesses at the scalp or source level.
One of the most studied tasks in relation to cognitive control in psychiatry is the continuous performance task (CPT; Dickinson et al. 2008). The cued version of the task (CPT-AX; Doehnert et al. 2010, 2008; McLoughlin et al. 2010, 2011; Valko et al. 2009) has the advantage of measuring two important components of cognitive control: attentional control (how to allocate attentional resources), via responses to ‘Go’ stimuli, and also response inhibition (monitoring performance in face of conflicts),via responses to “NoGo” stimuli. Deficits in both performance and ERP measures for the CPT-AX task, in particular in the amplitudes of the “Go N2/P3” complex and the corresponding “NoGo N2/P3” peaks aligned with response-initiating and response-inhibiting letter presentations, respectively, are consistently and strongly associated with psychiatric illness, including schizophrenia (Holmes et al. 2005; Lee and Park 2006; Salgado-Pineda et al. 2004), ASD (Lundervold et al. 2012; Tye et al. 2014; Wang et al. 2013) and ADHD (Albrecht et al. 2012; Banaschewski et al. 2004; Brandeis et al. 2006; Huang-Pollock et al. 2012; McLoughlin et al. 2010, 2011).
In the current study, we examined the relationship of two key components of cognitive control, namely the relationship of response execution and inhibition during the (CPT-AX) cued continuous performance task to the COMT val158met and DRD4 exon III VNTR polymorphisms. Previous publications based on this data set have already addressed the genetic modulation of motor postprocessing (Bender et al. 2012a), motor response variability (Bender et al. 2015), and visual postprocessing (Bender et al. 2012b) using statistical analysis at the sensor level of scalp ERPs. To more accurately characterise the complex cognitive sub-processes of cognitive control, we here increased the precision of these EEG measures by estimating brain activity at the source via decomposition of the data into maximally independent source processes using independent component analysis (ICA). ICA identifies component processes in the EEG data that are not only temporally near independent but typically also functionally independent in the sense that they exhibit more distinct response patterns to experimental events of interest than do the same measures applied to the raw channel data, consistent with the fact that scalp channel signals each sum potentials volume conducted to the scalp from a a wide variety of relevant and irrelevant brain and non-brain sources (McLoughlin et al. 2013) (Makeig et al. 1996, 1997).
ICA decomposition has been shown to yield event-related measures that share more genetic variance with behaviour than channel-based EEG measures (McLoughlin et al. 2014), to better characterize individual differences in schizophrenia and ADHD symptomology than scalp channel measures (Lenartowicz et al. 2014; McLoughlin et al. 2014; Rissling et al. 2014). Thus, in general ICA decomposition may enable a more precise characterization of spatiotemporally complex cortical network dynamics associated with cognitive control than equivalent measures applied directly to the recorded data channels.
Based on previous findings, we predicted that carriers of the COMT Met/Met allele will demonstrate improved neural function in the cortical source activities related to response execution and inhibition compared to other COMT alleles, and conversely that individuals with the DRD4 7-repeat will show impaired response execution and inhibition. We also consider the impact of the interaction effect between the two polymorphisms on the neural measures of cognitive control.
Materials and Methods
Participants
The current data analysis was conducted on the sample of the Mannheim Study of Children at Risk, a prospective longitudinal study of the outcome of early risk factors from infancy into adulthood (Laucht et al. 2000, 1997b). Children born between 1986 and 1988 were recruited from two obstetric and six pediatric hospitals of the Rhine-Neckar Region of Germany. Infants were included consecutively into the study according to a 2-factorial design intended to enrich and to control the risk status of the sample (full details of the sampling procedure have been reported previously Laucht et al. 1997a). As a result, approximately two-thirds of the study sample had experienced obstetric complications such as preterm birth, and about two-thirds of the families had psychosocial adversities such as marital discord or chronic difficulties. The current investigation included 174 healthy adolescents participating in the 15-year assessment for whom both genetic and 64-channel EEG data were available. Of the initial sample of 384 participants, 18 (4.7%) were excluded because of severe handicaps (neurological impairment or IQ/MQ < 70), 28 (7.3%) were drop-outs at age 15, 35 (9.1%) refused to take part in blood sampling or had incomplete genetic data, and from 43 (11.2%) no (or no reliable) EEG data were available. Intelligence had been assessed at the age of 11 years using the Culture Fair Test 20 (Cattell 1960; RH 1987); the motor quotient was determined at age 11 years by a short version of the Body Coordination Test for children KTK (Kiphardt 1974). 65 subjects (16.7%) were excluded from the current analysis due to a current psychiatric DSM-IV diagnosis. 21 subjects of the remaining 195 (10.8%) had to be excluded because they were not right-handed as indicated by a handedness index above + 60 in the Edinburgh Handedness Inventory (RC 1971). All subjects were free of psychoactive medication at the time of the recording and had a corrected visual acuity of 0.8 or higher. The study was approved by the ethics committee of the Medical Faculty of the University of Heidelberg/Mannheim. Written informed consent was obtained from all participants and their parents.
Task
Participants performed a computerized A-X version of the continuous performance test (CPT) constructed by doubling the number of trials of a common previous multicenter version (Banaschewski et al. 2003; Bender et al. 2007; van Leeuwen et al. 1998). A total of 800 black-colored capital letters were presented on a white background in the center of the computer screen for 150 ms each. The stimulus onset asynchrony (SOA) between letter presentations was 1600 ms. Whenever an ‘A’ was followed by an ‘X’ (i.e., whenever the letter sequence ‘AX’ was presented) subjects were asked to respond with a rapid right-hand button press of their index finger on the response pad. During testing, the ‘A’ was followed by an ‘X’ 80 times and by some other letter (distractor) 80 times; an ‘X’ without a preceding ‘A’ also occurred 80 times. Non-AX distractors were nine other letters of the alphabet (‘B’, ‘C’, ‘D’, ‘E’, ‘F’, ‘G’, ‘H’, ‘J’, ‘L’). Of these, a frequent distractor (‘H’) was presented 160 times and distractors ‘B’, ‘C’, ‘D’, ‘E’, ‘F’, ‘G’, ‘J’, and ‘L’ each 40 times. Here we present results of analysis of ERP responses to Go cue stimuli (‘X’ following an ‘A’) and NoGo cue stimuli (any other letter following an ‘A’).
EEG Recording and Preprocessing
Continuous 64-channel DC EEG data were recorded using Neuroscan Sympamps amplifiers (Neuroscan Inc., TX, USA). Sintered silver/silver chloride electrodes were positioned in an equidistant electrode cap montage (Easycap, FMS, Germany). Electrode impedances were kept below 10 k Ohm. The vertical electrooculogram (VEOG) was recorded with bipolar reference by electrodes 1 cm below and above the left eye. The horizontal electrooculogram (HEOG) was calculated by bipolar leads F99 and F109 next to the outer canthii. The recording reference was placed near the left mastoid. The sampling rate was 500 Hz per channel. An anti-aliasing low-pass filter with a cut-off frequency of 100 Hz was employed. The visual stimulation was presented by Gentask of the Neuroscan Stim software package. Reaction times were collected from response triggers from the response pad. EEG data processing was performed offline using the EEGLAB toolbox (v11.0.3.1b) (Delorme and Makeig 2004) for MATLAB (R2012a; The Mathworks, Inc., Natick, Massachusetts). Before analysis, the channel signals were re-referenced to average reference. We then applied a 1-Hz high-pass filter. Time points with any channel value larger than 200 μV in absolute value were rejected from the data and excluded from further analysis. In total, based on our EEG data preprocessing, we rejected the 0.03% of trial for the Go condition and 0.04% for the NoGo.
Genotyping
EDTA anticoagulated venous blood samples were collected. Leukocyte genomic deoxyribonucleic acid (DNA) was isolated with the Qiamp DNA extraction kit. Genotyping of the COMT single nucleotide polymorphism (SNP) was completed using TaqMan (SNP) Genotyping Assays. Genotyping of the DRD4 exon III VNTR polymorphism was performed using polymerase chain reaction according to Lichter et al. (1993; 14). All genotypes were scored independently by two raters who were blind to the presented data. A further 16 subjects were excluded from the genotype analysis due to noisy EEG data. In detail, the following genotype groups were formed: (1) DRD4 genotypes were classified into two groups according to the presence or absence of the 7r allele:7r (75) versus non-7r (132); (2) COMT: Val/Val (N = 41) versus Val/Met (N = 120) versus Met/Met (N = 46); and (3) COMT X DRD4: Val/Val 7r (N = 14) versus Val/Val non-7r (N = 27), Val/Met 7r (N = 45) versus Val/Met non-7r (N = 75), and Met/Met 7r (N = 16) versus Met/Met non-7r (N = 30).
EEG Data Analysis
We used adaptive mixture ICA (AMICA) (90, 91) to separate the multichannel data for each into maximally instantaneous independent component (IC) processes. Decompositions used the 64 available channels, removing channels determined to have bad contact (those with extended periods of low correlation with neighboring channels). The sampling rate was 500 Hz. Epochs were 2 s, extending from 800 ms pre-stimulus to 1200 ms post-stimulus. We computed an equivalent dipole model for each IC scalp topography using a template four-layer adult boundary element method head model implemented in the DIPFIT toolbox for MATLAB (92). To optimally combine IC information from different participants, we applied a probabilistic multi-subject inference approach, measure projection analysis (MPA) (93), to event-related potential (ERP) waveforms time-locked to Go and NoGo stimuli, respectively.
Measure projection involves projecting the selected measure into a 3-D grid of voxels filling the template brain volume. For each brain-based IC, the measure is then ‘projected’ to voxels within a spherical region centered on the IC equivalent dipole location. MPA then searches the 3-D voxel grid for voxels for which the projected measures at nearby ICs are (1) sufficiently numerous, and (2) exhibit statistically significant consistency. Here, Measure Projection was applied to Go and NoGo stimulus-locked IC ERP waveforms using EEGLAB default parameter values (significance level, p = .01; maximum domain exemplar correlation, r = 0.7). For each voxel, the projected measures of all ICs whose equivalent dipole location is within the smoothing radius are then summed with weights inversely proportional to the distance of the IC equivalent dipole location from the central voxel. At each voxel, the projected measures for all the brain ICs across the subjects are then normalized.
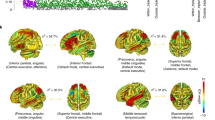
To simplify the analysis of projected source measure values in the remaining set of voxels (the measure consistency subspace), MPA then separates them into several distinguishable spatial domains using threshold-based affinity propagation clustering as described in detail in (93). Affinity propagation is based on a similarity matrix of pairwise correlations between the projected measures at each voxel position. The method automatically determines an appropriate number of voxel clusters (below referred to as spatial domains) based on the maximum allowed correlation between cluster exemplars, automatically increasing the number of clusters until any other potential cluster exemplar becomes too similar to one of the existing exemplars (93). This approach identified seven spatial domains for the source-resolved ERP data (Fig. 1). Two domains of interest (DOIs) centered in frontal cortex and the parietal/temporal cortex (Domains 1 and 2; Fig. 2) produced prominent P3 and N2 peaks in group grand mean ERPs to Go cue stimuli and were selected for further analysis. ERPs from other identified domains included smaller or ambiguous P3 and N2 peak features and were not considered further.
Measure projection domains. We selected the central/parietal Domain 1 (shown in red) and the superior-frontal Domain 2 (shown in yellow) for further analysis as these domains are associated with attention and executive function (Castellanos and Proal 2012; Cortese et al. 2012) and yielded clear N2-P3 type ERPs (the ERPs associated with the other domains did not appear to contribute to the N2 and P3 ERPs)
The two selected Measure Projection domains: parietal/temporal cortical source Domain 1 (left, posterior view) and frontal cortex source Domain 2 (right, anterior view) and their associated group grand mean ERP responses to Go and NoGo cue stimuli. ‘P3’ peaks (350–400 ms) were largest in the centro-parietal domain responses, while ‘N2’ peaks (near 350 ms) are largest in the frontal source domain responses. Y-axis unit: peak projected RMS uV across all scalp channels
Statistical Analyses
For statistical analysis of group differences, genotype groups were compared in terms of peak amplitudes and/or peak area under the curve in trial-average ERPs time-locked to Go and NoGo stimuli where the ‘Go’ stimuli were letter ‘X’ stimuli following letter ‘A’ cue presentations (cueing a button press response), and the NoGo stimuli were any distractor (response-inhibiting) letter following an ‘A’ (i.e., a false alarm condition in which the subject had to inhibit their partially anticipated response). Groups were compared based on their Go and NoGo 200–400 ms post-stimulus ‘P3’ peak amplitudes (Domain 1; Fig. 2). For statistical analysis we used maximal peak amplitude for the Go cue response P3 and the area under the curve for the NoGo cue response P3 as there was no clear peak for the latter. The N2 measure was the maximal negative peak value in the Domain 2 ERP waveforms between 300 and 400 ms post-stimulus (Go and NoGo; Domain 2; Fig. 2). Performance measures were number of commission errors, omission errors, mean reaction time to target stimulus presentations (RTM, i.e., mean latency of responding (in ms) following target onsets) and within-subject variability in target reactions times (RTSD). We ran multivariate analyses of variance for each ERP measure (Go and NoGo conditions) using Bonferroni correction for posthoc tests. Effect sizes were calculated by converting eta-squared (\({\eta }^{2}\)) to Cohen’s \(d\) using the formula: \(d=\sqrt{\frac{{\eta }^{2}}{1-{\eta }^{2}}} \sqrt{2k},\) where \(k\) is the number of groups (Cohen 1988).
Results
Performance Data
Performance data is presented in Table 1. No main effect of COMT genotype emerged for any of the performance variables [RTM: F(1,207) = 0.52, p = .60, d = 0.19; RTSD: F(1,207) = 0.60, p = .55, d = 0.17, commission errors: F(1,207) = 0.16, p = .85, d = 0.13; omission errors: F(1,207) = 0.32, p = .73, d = 0.16]. Nor was there any effect of DRD4 genotype on any of the performance variables [RTM: F(1,207) = 0.57, p = .45, d = 0; RTSD: F(1,207) = 0.15, p = .70, d = 0; commission errors: F(1,207) = 0.83, p = .36, d = 0.08; omission errors: F(1,207) = 0.41, p = .52, d = 0]. Similarly, no interaction effect emerged for any performance variables [NoGo P3; Go N2; NoGo N2; RTM, RTSD, commission errors; omission errors: all p > .37, all d < 0.25].
ERP ICA Source Findings
ERP data is presented in Table 2.
COMT
A main effect for COMT genotype on P3 amplitude was obtained for the NoGo ERP [F(2, 207) = 3.52, p = .03, d = 0.46] but not for the Go ERP [F(2, 207) = 1.86, p = .16, d = 0.33] (MP domain 1). Posthoc t-tests indicated no difference between the Val/Val and Val/Met groups (p = .10, d = 0) in NoGo P3 amplitude; however the Met/Met genotype group had a significantly smaller NoGo P3 mean amplitude compared to either the Val/Val (p = .04, d = 0.45) or the Val/Met (p = .01, d = 0.44) genotype groups. See Figs. 3 and 4. No main effect emerged for COMT genotype on the N2 amplitude for either condition [Go: (F(2, 207) = 0.66, p = .52, d = 0.19; NoGo: F(2, 207) = 0.71, p = .49, d = 0.46].
P3 peak amplitudes in ERPs to NoGo stimuli for parietal/temporal source domain (Domain 1) show a main effect of COMT allele. Y-axis unit: as in Fig. 2
DRD4
We found a significant effect of DRD4 genotype on Go P3 amplitude [F(2,207) = 5.83, p = .017, d = 0.42], which was smaller in subjects with the DRD4 7-repeat than in those without it. No evidence emerged for an effect of DRD4 genotype on NoGo P3 mean amplitude [NoGo: F(2, 207) = 1.45, p = .23, d = 0.21]. Similarly, no effect emerged for N2 amplitude in either condition [Go: F(2, 207) = 1.48, p = .23, d = 0.17, NoGo: F(2, 207) = 0.150, p = .70, d = 0.11].
COMT × DRD4
An interaction was detected between the COMT and DRD4 polymorphisms for Go response P3 amplitude [F(2, 207) = 3.99, p = .02, d = 0.49]. Post hoc analyses indicated that having the DRD4 genotype reduced Go response P3 amplitude in Val/Val (p = .02, d = 0.80) and Met/Met (p = .03, d = 0.66), but not in Val/Met carriers (p = .43, d=-.14) (Figs. 5, 6).
Grand mean group ERP responses to Go cue stimuli for the parietal-temporal source Domain 1 (see Fig. 2) reveal an interaction between COMT and DRD4genotypes on amplitude of the P3 peak. Y-axis unit: as in Fig. 2. M/M Met/Met polymorphism; V/M Val/Met; V/V Val/Val; non-7r non 7-repeat; 7r 7-repeat polymorphism groups
ERP Channel Findings
The same statistical analysis was run on the raw EEG channel data at the scalp channels where the ERP amplitude was maximal. Eye blink components were identified by ICA and removed; the remaining source data were subsequently backprojected to these channels. The amplitudes of the scalp P3 peaks were measured at scalp channel Pz; the N2 peak amplitudes were measured at scalp channel Cz. Similar to the findings at the source level, a main effect for COMT genotype was obtained for Nogo P3 mean amplitude at channel Pz [F(2, 207) = 3.29, p = .04, d = 0.45], but not for Go P3 peak amplitude [F(2, 207) = 2.06, p = .13, d = 0.35]. A significant main effect of DRD4 on P3 peak amplitude at Pz was found for the NoGo condition (F(2, 207) = 6.74, p = .01, d = 0.45) but not for the Go condition (F(2, 207) = 2.74, p = .10, d = 0.28), contrary to the source-resolved findings. No effect of DRD4 on N2 peak amplitude emerged at Cz in either condition [Go: F(2, 207) = 0.41, p = .52, d = 0.11; NoGo: F(2, 207) = 0.82, p = .37, d = 0.16]. No interaction emerged for any of the other channel or performance variables [N2 amplitude at channel Cz in either condition; Go P3; NoGo P3; RTM; RTSD; commission errors; omission errors: all p > .36, all d < 0.15]. No interaction effects were detected between COMT and DRD4 for any of the channel measures [Go P3 amplitude: NoGo P3; Go N2; NoGo N2; all p > .37, all d < 0.25].
Discussion
In this targeted candidate gene analysis, we identified several associations between polymorphisms of dopamine system genes and ERP indices of cognitive control during the cued continuous performance task CPT-AX. Specifically, we found that the homozygous Met allele of the COMT genotype was associated with smaller mean P3 amplitude in the IC ERP time-locked to Nogo stimuli and further that individuals with the 7-repeat polymorphism of DRD4 had a smaller peak IC-P3 to Go stimuli. Furthermore, an interaction effect between COMT and DRD4 emerged, indicating a smaller IC-derived Go-P3 in those with the 7-repeat if they also had the homozygous Val/Val polymorphism or the homozygous Met/Met polymorphism, but not in those with the heterozygous Val/Met polymorphism. In contrast to these findings, there was no association between any of the selected polymorphisms and performance measures or the IC-N2 measures.
The overall pattern of task–related ERP activity in our study was consistent with the fronto-parieto midline prominent N2-P3 peak complex observed in tasks that require attentional and cognitive control (Castellanos and Proal 2012; Jaspar et al. 2014). Due to proposed site of action of the COMT enzyme, most of the research on COMT has focused on prefrontally-mediated cognition. Our study instead found that Met allele homozygotes exhibited a smaller ERP measure of response inhibition for a parietal/temporal source domain, but did not identify a significant relationship between COMT and activity in prefrontal cortex. While, COMT alters enzyme activity in prefrontal cortex, and has been strongly associated with activation in frontal brain regions during task performance, the expression of this enzyme is widespread and relatively uniform within the human brain (Hong et al. 2014). Recent findings indicate that the effect of the COMT val108/158met polymorphism extends beyond the PFC and has different effects on brain activity and structure in other regions (Hong et al. 2015), and may specifically include parietal activity during inhibitory control (Van Rooij et al. 2015). These findings indicate that COMT-related inhibitory brain activity is not confined to prefrontal regions.
That the COMT Met/Met allele was associated with an impairment in a neural index of response inhibition may be viewed as surprising, given that the Met allele has been associated with improved performance in executive function and cognitive tests (Cahoy et al. 2008; Freeman and Rowitch 2013; Kiang et al. 2009; Wynn et al. 2010). However, the role of dopamine in the brain and its relationship with the neural network underlying cognitive control is complex (Durstewitz and Seamans 2008; Goldman-Rakic et al. 2000). The dual state or U shaped theories of dopamine regulation (Durstewitz and Seamans 2008; Meyer-Lindenberg and Weinberger 2006) predict that homozygous Met carriers have high tonic dopamine levels in the prefrontal cortex (PFC) and thus optimal levels of dopamine for tasks that require cognitive stability or maintenance, including working memory and competing programs (Nolan et al. 2004), while Val carriers have high phasic dopamine levels in the PFC, and are thus more efficient when cognitive flexibility is required, such as during rapid updating or task switching (Colzato et al. 2010; Drabant et al. 2006; Nolan et al. 2004). Our finding is largely consistent with studies examining the role of COMT that have found that those with the Met/Met polymorphism have impaired response inhibition performance in comparison to Val carriers (Weiss et al. 2014). Our findings are in further agreement with studies that show that the homozygous Met allele is associated with behavioural measures of impulsivity (Soeiro-De-Souza et al. 2013; Stipursky et al. 2011), particularly with a failure to plan ahead (Soeiro-De-Souza et al. 2013) and poorer delayed discounting (Stipursky et al. 2011).
We confirmed our prediction that the DRD4 7-repeat allele was associated with abnormalities in neural indices of attentional control. Previous studies have implicated this polymorphism in attention-related problems in both typically developing children (Auerbach et al. 2001; Schmidt et al. 2002) and in clinical samples of children with ADHD (Faraone et al. 2005). The attention-related EEG activity differences for the parietal/temporal domain in this study are in line with multiple functional imaging studies that showed that attentional control is associated with the activity of a network of brain regions, including the parietal cortex (Buschman and Miller 2007), which has specific involvement in sustained (Coull et al. 1996) and orienting attention (Yantis et al. 2002).
Moreover, we found that the relationship between DRD4 and attentional control (IC-go-P3) varied depending on COMT genotype. This pattern of an epistatic interaction between DRD4 and COMT for attentional control is somewhat consistent with the proposed inverted U-shaped curve for the optimal range of dopamine availability for task performance, according to which too little or too much dopamine is disruptive and impairs functioning of the system (Bruggemann et al. 2013; Congdon et al. 2009; Durstewitz and Seamans 2008; Takahashi et al. 2011). Carriers of the DRD4 7-repeat allele with either of the homozygous COMT polymorphisms presumably had too high levels of dopamine availability for optimal attentional control and exhibited an attenuated pattern of activation to Go stimuli in the CPT-AX. Whereas individuals without the 7-repeat and either of the homozygous COMT polymorphisms may have had optimal levels of dopamine availability. In contrast, there was no influence of the DRD4 7-repeat allele on individuals with the heterozygous Val/Met allele. Dopamine is proposed to modulate the response of neural networks by suppressing spontaneous background firing and thus increasing signal-to-noise ratio and enhancing the task-specific response (Winterer et al. 2006). Our study suggests that both high and low dopaminergic states (Met/Met and Val/Val, respectively) may be compensated by increased D4 function and thus enhancing neural tuning in attentional networks.
A limitation of our study is the candidate gene approach. The selected polymorphisms will not cover all genetic variation within the examined neural circuits. We chose alleles with known functional effects and/or previously reported effects on relevant phenotypes instead of examining a larger set of SNPs tagging the major haplotypes within dopaminergic genes. Therefore, we covered only a small portion of the total dopamine signaling pathway. Already there are many other genes known to be involved in this signaling pathway (Beaulieu and Gainetdinov 2011) and a candidate gene approach will miss most of these signals. Replication is required and the relationship between source localisation measures in EEG and advanced genetic measures, including polygenic scores should be explored.
Despite evidence that psychiatric disorders have genetic etiology (Kendler), there has been a lack of identification of genetic risk factors that reliably associate with psychopathology ({Casey et al. 2013). Psychiatric disorders are likely to involve multiple brain systems and patients may differ in the extent to which processing in these systems is affected so that the biological roots within a clinical diagnosis may vary substantially (Miller 2010). Targeted analysis using functional neuroimaging measures within genetic designs could aid the identification of neural circuits affected in DSM diagnoses to more precisely diagnose and treat psychopathology (Insel and Cuthbert 2015).
No relationship between cognitive performance measures and the genetic markers was found. It may be that the infrequency of required response and inhibition in the CPT-AX (respectively, 10% of stimuli) does not provide enough data points to accurately describe these processes for use in genetic investigations as genetic effects may have relatively small effect sizes compared to, for example, group differences. Genetic effects may be more apparent in information-rich time series data at the neural level than detection via infrequent button presses at the behavioural level. This is reflected in the small effect sizes for all of the cognitive performance measures. Research focusing on overt behavioral correlates of genetic effects on attention and inhibition thus typically uses tasks that require more frequent responding, which may provide greater behavioral resolution of the cognitive process.
Although polymorphisms of COMT and DRD4 had been linked to EEG phenotypes (Loo et al. 2010; Solís-Ortiz et al. 2015), these potential links had not yet been investigated within tasks examining the neurophysiological indices of frontostriatal and parietal attentional networks. Our study aimed to investigate the relationship between COMT and DRD4 polymorphisms and ICA source-resolved brain activity during a cognitive control task, and our findings suggest higher genetic penetrance for IC-derived EEG measures of cognitive control than traditional channel based measures. The associations between these dopamine system gene function and ERP measures to be stronger at the source than at the scalp level, particularly for the COMT x DRD4 interaction; however our results remain correlational. They do not exclude mediating mechanisms, and should not be construed to suggest one-to-one relation between these gene functions and ERP source component measures.
The improved functional and anatomic separation of the cortical signal sources of EEG data produced by ICA decomposition may give measures that may be more informative for genetic studies of brain function. This may be because scalp data channels are each source signal admixtures, so that the effective signal-to-noise ratio of the ICA-separated cortical source activities is under favorable circumstances much higher than for the scalp channel signals (45). This result is consistent with the understanding that for biophysical reasons each scalp channel recording sums activities generated in many places in cortex, whereas many independent component processes separated from the data by ICA decomposition are compatible with an origin in just one cortical area or patch. Thus, neuroimaging methods, including source-based EEG measures, may be more powerful for unravelling gene-brain behavioural relationships than scalp channel-based EEG measures (McLoughlin et al. 2013; Weinberger et al. 2001).
References
Albrecht B et al (2012) Familiality of neural preparation and response control in childhood attention deficit-hyperactivity disorder. Psychol Med. doi:10.1017/S003329171200270X
Albrecht B et al (2014) Genetics of preparation and response control in ADHD: the role of DRD4 and DAT1. J Child Psychol Psychiatry 55:914–923
Anokhin AP, Heath AC, Myers E (2004) Genetics, prefrontal cortex, and cognitive control: a twin study of event-related brain potentials in a response inhibition task. Neurosci Lett 368:314–318
Auerbach JG, Benjamin J, Faroy M, Geller V, Ebstein R (2001) DRD4 related to infant attention and information processing: a developmental link to ADHD? Psychiatr Genet 11:31–35
Banaschewski T, Brandeis D, Heinrich H, Albrecht B, Brunner E, Rothenberger A (2003) Association of ADHD and conduct disorder–brain electrical evidence for the existence of a distinct subtype. J Child Psychol Psychiatry 44:356–376
Banaschewski T, Brandeis D, Heinrich H, Albrecht B, Brunner E, Rothenberger A (2004) Questioning inhibitory control as the specific deficit of ADHD–evidence from brain electrical activity. J Neural Transm 111:841–864
Banaschewski T, Becker K, Scherag S, Franke B, Coghill D (2010) Molecular genetics of attention-deficit/hyperactivity disorder: an overview. Eur Child Adolesc Psychiatry 19:237–257
Barkley RA, Smith KM, Fischer M, Navia B (2006) An examination of the behavioral and neuropsychological correlates of three ADHD candidate gene polymorphisms (DRD4 7+, DBH TaqI A2, and DAT1 40 bp VNTR) in hyperactive and normal children followed to adulthood American. J Med Genet Part B 141:487–498
Beaulieu J-M, Gainetdinov RR (2011) The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev 63:182–217
Bellgrove MA, Hawi Z, Lowe N, Kirley A, Robertson IH, Gill M (2005) DRD4 gene variants and sustained attention in attention deficit hyperactivity disorder (ADHD): effects of associated alleles at the VNTR and –521. SNP Am J Med Genet B 136B:81–86
Bender S, Weisbrod M, Resch F, Oelkers-Ax R (2007) Stereotyped topography of different elevated contingent negative variation components in children with migraine without aura points towards a subcortical dysfunction. Pain 127:221–233. doi:10.1016/j.pain.2006.08.017
Bender S et al (2012a) Dopamine inactivation efficacy related to functional DAT1 and COMT variants influences motor response evaluation. PloS One 7:e37814
Bender S et al (2012b) Time-resolved influences of functional DAT1 and COMT variants on visual perception and post-processing. PloS One 7:e41552
Bender S et al (2015) Variability of single trial brain activation predicts fluctuations in reaction time. Biol Psychol 106:50–60
Bertolino A et al (2004) Interaction of COMT Val108/158 Met genotype and olanzapine treatment on prefrontal cortical function in patients with schizophrenia. Am J Psychiatry 161:1798–1805
Biederman J, Kim JW, Doyle AE, Mick E, Fagerness J, Smoller JW, Faraone SV (2008) Sexually dimorphic effects of four genes (COMT, SLC6A2, MAOA, SLC6A4) in genetic associations of ADHD: a preliminary study American. J Med Genet Part B 147:1511–1518
Blasi G et al (2005) Effect of catechol-O-methyltransferase val158met genotype on attentional control. J Neurosci 25:5038–5045
Brandeis D, Banaschewski T, Albrecht B, Rothenberger A, Steinhausen H (2006) Brainmapping of attention and inhibition in ADHD sib pairs using CPT variants European Network on Hyperkinetic Disorders (EUNETHYDIS),
Bruggemann JM, Stockill HV, Lenroot RK, Laurens KR (2013) Mismatch negativity (MMN) and sensory auditory processing in children aged 9–12 years presenting with putative antecedents of schizophrenia. Int J Psychophysiol. doi:10.1016/j.ijpsycho.2013.05.008
Buschman TJ, Miller EK (2007) Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science 315:1860–1862
Cahoy JD et al (2008) A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci 28:264–278. doi:10.1523/JNEUROSCI.4178-07.2008
Casey C, Craddock BJ, Cuthbert N, Hyman BN, Lee SE, Ressler FS KJ (2013) DSM-5 and RDoC: progress in psychiatry research? Nat Rev Neurosci 14:810–814
Castellanos FX, Proal E (2012) Large-scale brain systems in ADHD: beyond the prefrontal-striatal model. Trends Cogn Sci 16:17–26
Cavanagh JF, Zambrano-Vazquez L, Allen JJ (2012) Theta lingua franca: a common mid-frontal substrate for action monitoring processes. Psychophysiology 49:220–238. doi:10.1111/j.1469-8986.2011.01293.x
Cohen J (1988) Statistical power analysis for the behavioral sciences, 2nd edn. Lawrence Erlbaum, New Jersey
Colzato LS, Waszak F, Nieuwenhuis S, Posthuma D, Hommel B (2010) The flexible mind is associated with the catechol-O-methyltransferase (COMT) Val 158 Met polymorphism: evidence for a role of dopamine in the control of task-switching. Neuropsychologia 48:2764–2768
Committee PGCC (2009) Genomewide association studies: history, rationale, and prospects for psychiatric disorders. Am J Psychiatry 166:540–556
Congdon E, Constable RT, Lesch KP, Canli T (2009) Influence of SLC6A3 and COMT variation on neural activation during response inhibition. Biol Psychol 81:144–152
Cools R (2008) Role of dopamine in the motivational and cognitive control of behavior. Neuroscientist 14:381–395
Cortese S, Kelly C, Chabernaud C, Proal E, Di Martino A, Milham MP, Castellanos FX (2012) Toward systems neuroscience of ADHD: a meta-analysis of 55 fMRI studies. Am J Psychiatry 169:1038–1055. doi:10.1176/appi.ajp.2012.11101521
Coull J, Frith C, Frackowiak RSJ, Grasby P (1996) A fronto-parietal network for rapid visual information processing: a PET study of sustained attention and working memory. Neuropsychologia 34:1085–1095
Delorme A, Makeig S (2004) EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods 134:9–21
DeYoung CG et al (2010) Variation in the catechol-O-methyltransferase Val158Met polymorphism associated with conduct disorder and ADHD symptoms among adolescent male delinquents. Psychiatric Genet 20:20
Diamond A, Briand L, Fossella J, Gehlbach L (2014) Genetic and neurochemical modulation of prefrontal cognitive functions in children. Am J Psychiatry 161:125–132
Dickinson D, Elvevåg B (2009) Genes, cognition and brain through a COMT lens. Neuroscience 164:72–87
Dickinson D, Ragland JD, Gold JM, Gur RC (2008) General and specific cognitive deficits in schizophrenia: Goliath defeats David? Biol Psychiatry 64:823–827. doi:10.1016/j.biopsych.2008.04.005
Doehnert M, Brandeis D, Straub M, Steinhausen HC, Drechsler R (2008) Slow cortical potential neurofeedback in attention deficit hyperactivity disorder: is there neurophysiological evidence for specific effects? JNeural Transm 115:1445–1456
Doehnert M, Brandeis D, Imhof K, Drechsler R, Steinhausen HC (2010) Mapping attention-deficit/hyperactivity disorder from childhood to adolescence—no neurophysiologic evidence for a developmental lag of attention but some for inhibition BiolPsychiatry. 67:608–616
Drabant EM et al (2006) Catechol O-methyltransferase val158met genotype and neural mechanisms related to affective arousal and regulation. Arch Gen Psychiatry 63:1396–1406
Durstewitz D, Seamans JK (2008) The dual-state theory of prefrontal cortex dopamine function with relevance to catechol-O-methyltransferase genotypes and schizophrenia. Biol Psychiatry 64:739–749
Ehlis A-C, Reif A, Herrmann MJ, Lesch K-P, Fallgatter AJ (2007) Impact of catechol-O-methyltransferase on prefrontal brain functioning in schizophrenia spectrum disorders. Neuropsychopharmacology 32:162–170
Eroglu C, Barres BA (2010) Regulation of synaptic connectivity by. glia Nature 468:223–231. doi:10.1038/nature09612
Fallgatter AJ, Brandeis D, Strik WK (1997) A robust assessment of the NoGo-anteriorisation of P300 microstates in a cued continuous performance. Test Brain Topogr 9:295–302
Faraone SV, Doyle AE, Mick E, Biederman J (2001) Meta-analysis of the association between the 7-repeat allele of the dopamine D(4) receptor gene and attention deficit hyperactivity disorder. Am J Psychiatry 158:1052–1057
Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, Sklar P (2005) Molecular genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry 57:1313–1323
Freeman MR, Rowitch DH (2013) Evolving concepts of gliogenesis: a look way back and ahead to the next 25 years. Neuron 80:613–623. doi:10.1016/j.neuron.2013.10.034
Friedman NP, Miyake A, Young SE, DeFries JC, Corley RP, Hewitt JK (2008) Individual differences in executive functions are almost entirely genetic in origin. J Exp Psychol Gen 137:201
Gallinat J et al (2003) Association of the G1947A COMT (Val 108/158 Met) gene polymorphism with prefrontal P300 during information processing. Biol Psychiatry 54:40–48
Goldberg TE, Weinberger DR (2004) Genes and the parsing of cognitive processes. Trends Cogn Sci 8:325–335
Goldman-Rakic PS, Muly EC III, Williams GV (2000) D 1 receptors in prefrontal cells and circuits. Brain Res Rev 31:295–301
Gottesman II, Gould TD (2003) The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry 160: 636–645
Hawi Z, Cummins TD, Tong J, Johnson B, Lau R, Samarrai W, Bellgrove MA (2015) The molecular genetic architecture of attention deficit hyperactivity. disorder Mol Psychiatry 20:289–297. doi:10.1038/mp.2014.183
Holmes AJ, MacDonald A, Carter CS, Barch DM, Stenger VA, Cohen JD (2005) Prefrontal functioning during context processing in schizophrenia and major depression: an event-related fMRI study. Schizophrenia Res 76:199–206
Hong S-B et al (2014) Connectomic disturbances in attention-deficit/hyperactivity disorder: a whole-brain tractography. Anal Biol Psychiatry 76:656–663
Hong SB et al (2015) COMT genotype affects brain white matter pathways in attention-deficit/hyperactivity disorder. Hum Brain Mapp 36:367–377
Huang-Pollock CL, Karalunas SL, Tam H, Moore AN (2012) Evaluating vigilance deficits in ADHD: a meta-analysis of CPT performance. J Abnorm Psychol 121:360
Insel TR (2014) The NIMH research domain criteria (RDoC) project: precision medicine for psychiatry American. J Psychiatry 171:395–397
Insel TR, Cuthbert BN (2009) Endophenotypes: bridging genomic complexity and disorder heterogeneity. Biol Psychiatry 66:988–989. doi:10.1016/j.biopsych.2009.10.008
Insel TR, Cuthbert BN (2015) Brain disorders? Precisely. Science 348:499–500
Jaspar M, Manard M, Dideberg V, Bours V, Maquet P, Collette F (2014) Influence of COMT genotype on antero-posterior cortical functional connectivity underlying interference resolution Cereb Cortex. doi:10.1093/cercor/bhu188
Johnson KA et al. (2008) Absence of the 7-repeat variant of the DRD4 VNTR is associated with drifting sustained attention in children with ADHD but not in controls. Am J Med Genet B 147B:927–937
Joober R et al (2002) Catechol-O-methyltransferase Val-108/158-Met gene variants associated with performance on the Wisconsin card sorting test. Arch Gen Psychiatry 59:662–663
Kendler KS, Eaves LJ (2005) Psychiatric genetics (review of Psychiatry). American Psychiatric Association, Arlington, VA
Kiang M, Braff DL, Sprock J, Light GA (2009) The relationship between preattentive sensory processing deficits and age in schizophrenia patients. Clin Neurophysiol 120:1949–1957. doi:10.1016/j.clinph.2009.08.019
Kiphardt EJ SF (1974) Körperkoordinationstest für Kinder (KTK). Beltz, Weinheim
Konrad K et al (2010) Familiality and molecular genetics of attention networks in ADHD American. J Med Genet Part B 153:148–158
Kramer UM et al (2007) The impact of catechol-O-methyltransferase and dopamine D4 receptor genotypes on neurophysiological markers of. performance monitoring. J Neurosci 27:14190–14198
Lachman HM, Papolos DF, Saito T, Yu Y-M, Szumlanski CL, Weinshilboum RM (1996) Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenet Genom 6:243–250
Laucht M, Esser G, Schmidt MH (1997a) Developmental outcome of infants born with biological and psychosocial risks. J Child Psychol Psychiatry 38:843–853
Laucht M, Esser G, Schmidt MH (1997b) Developmental outcome of infants born with biological and psychosocial risks. J Child Psychol Psychiatry 38:843–853
Laucht M et al (2000) Behavioral sequelae of perinatal insults and early family adversity at 8 years of age. J Am Acad Child Adolesc Psychiatry 39:1229–1237
Laurens KR, Hodgins S, Mould GL, West SA, Schoenberg PL, Murray RM, Taylor EA (2010) Error-related processing dysfunction in children aged 9 to 12 years presenting putative antecedents of schizophrenia. Biol Psychiatry 67:238–245. doi:10.1016/j.biopsych.2009.07.030
Lee J, Park S (2006) The role of stimulus salience in CPT-AX performance of schizophrenia patients. Schizophr Res 81:191–197
Lenartowicz A et al (2014) Electroencephalography correlates of spatial working memory deficits in attention-deficit/hyperactivity disorder: vigilance, encoding, and maintenance. J Neurosci 34:1171–1182
Li D, Sham PC, Owen MJ, He L (2006) Meta-analysis shows significant association between dopamine system genes and attention deficit hyperactivity disorder. HumMolGenet 15:2276–2284
Lichter JB, Barr CL, Kennedy JL, Van Tol HH, Kidd KK, Livak KJ (1993) A hypervariable segment in the human dopamine receptor D4 (DRD4) gene. Hum Mol Genet 2:767–773
Liu B, Song M, Li J, Liu Y, Li K, Yu C, Jiang T (2010) Prefrontal-related functional connectivities within the default network are modulated by COMT val158met in healthy young adults. J Neurosci 30:64–69
Loo SK et al (2010) Familial clustering and DRD4 effects on electroencephalogram measures in multiplex families with attention deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 49:368–377
Lundervold AJ, Stickert M, Hysing M, Sørensen L, Gillberg C, Posserud M-B (2012) Attention deficits in children with combined autism and adhd: a cpt study. J Atten Disord. doi:10.1177/1087054712453168
Luu P, Tucker DM, Makeig S (2004) Frontal midline theta and the error-related negativity: neurophysiological mechanisms of action regulation ClinNeurophysiol. 115:1821–1835
Macare C, Meindl T, Nenadic I, Rujescu D, Ettinger U (2014) Preliminary findings on the heritability of the neural correlates of response inhibition. Biol Psychol 103:19–23
Makeig S BA, Jung T-P, Sejnowski TJ (1996) Independent component analysis of electroencephalographic data. In: Touretzky MMaMH D (ed) Advances in Neural Information Processing Systems. MIT Press, Cambridge MA, pp 145–151
Makeig S, Jung TP, Bell AJ, Ghahremani D, Sejnowski TJ (1997) Blind separation of auditory event-related brain responses into independent components ProcNatlAcadSciUSA. 94:10979–10984
McLoughlin G, Albrecht B, Banaschewski T, Rothenberger A, Brandeis D, Asherson P, Kuntsi J (2010) Electrophysiological evidence for abnormal preparatory states and inhibitory processing in adult ADHD. Behav Brain Funct 6:66. doi:10.1186/1744-9081-6-66
McLoughlin G, Asherson P, Albrecht B, Banaschewski T, Rothenberger A, Brandeis D, Kuntsi J (2011) Cognitive-electrophysiological indices of attentional and inhibitory processing in adults with ADHD: familial effects Behavioral and Brain Functions 7
McLoughlin G, Makeig S, Tsuang MT (2013) In search of biomarkers in psychiatry: EEG-based measures of brain function. Am J Med Genet B. doi:10.1002/ajmg.b.32208
McLoughlin G, Palmer JA, Rijsdijk F, Makeig S (2014) Genetic overlap between evoked frontocentral theta-band phase variability, reaction time variability, and attention-deficit/hyperactivity disorder symptoms in a twin study. Biol Psychiatry 75:238–247. doi:10.1016/j.biopsych.2013.07.020
Meyer-Lindenberg A, Weinberger DR (2006) Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nat Rev Neurosci 7:818–827
Millan MJ et al (2012) Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov 11:141–168
Miller G (2010) Psychiatry. Beyond DSM: seeking a brain-based classification of mental illness. Science 327:1437. doi:10.1126/science.327.5972.1437
Nieoullon A (2002) Dopamine and the regulation of cognition and attention. Prog Neurobiol 67:53–83
Nolan KA, Bilder RM, Lachman HM, Volavka J (2004) Catechol O-methyltransferase Val158Met polymorphism in schizophrenia: differential effects of Val and Met alleles on cognitive stability and flexibility American. J Psychiatry 161:359–361
O’Sullivan SS, Evans AH, Lees AJ (2009) Dopamine Dysregulation Syndrome. CNS Drugs 23:157–170
Ogg RJ, Zou P, Allen DN, Hutchins SB, Dutkiewicz RM, Mulhern RK (2008) Neural correlates of a clinical continuous performance test. Magn Reson Imaging 26:504–512. doi:10.1016/j.mri.2007.09.004
Organization WH (2014) Preventing suicide: a global imperative. World Health Organization, Geneva
Plewnia C, Zwissler B, Längst I, Maurer B, Giel K, Krüger R (2013) Effects of transcranial direct current stimulation (tDCS) on executive functions: influence of COMT Val/Met. Polymorph Cortex 49:1801–1807
Ptacek R, Kuzelova H, Stefano GB (2011) Dopamine D4 receptor gene DRD4 and its association with psychiatric disorders. Med Sci Rev 17:RA215–RA220
Cattell RB (1960) Culture fair intelligence test, scale 2. 3rd edn. Institute for Personality and Ability Testing., Champaign
RC O (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113
RH W (1987) Grundintelligenztest CFT 20. Hogrefe, Göttingen
Rissling AJ, Miyakoshi M, Sugar CA, Braff DL, Makeig S, Light GA (2014) Cortical substrates and functional correlates of auditory deviance processing deficits in schizophrenia. Neuroimage Clin 6:424–437
Royall DR et al (2002) Executive control function: a review of its promise and challenges for clinical research. A report from the Committee on Research of the American Neuropsychiatric Association The. J Neuropsychiatry Clin Neurosci 14:377–405
Salgado-Pineda P, Junqué C, Vendrell P, Baeza I, Bargalló N, Falcón C, Bernardo M (2004) Decreased cerebral activation during CPT performance: structural and functional deficits. Schizophrenic Patients Neuroimage 21:840–847
Schmidt LA, Fox NA, Rubin KH, Hu S, Hamer DH (2002) Molecular genetics of shyness and aggression in preschoolers. Personality Individ Differ 33:227–238
Soeiro-De-Souza MG, Stanford MS, Bio DS, Machado-Vieira R, Moreno RA (2013) Association of the COMT Met158 allele with trait impulsivity in healthy young adults. Mol Med Rep 7:1067–1072
Solís-Ortiz S, Pérez-Luque E, Gutiérrez-Muñoz M (2015) Modulation of the COMT Val158Met polymorphism on resting-state EEG power frontiers. Front Hum Neurosci. doi:10.3389/fnhum.2015.00136
Stefanis NC, Van Os J, Avramopoulos D, Smyrnis N, Evdokimidis I, Hantoumi I, Stefanis CN (2004) Variation in catechol-O-methyltransferase val158 met genotype associated with schizotypy but not cognition: a population study in 543 young men. Biol Psychiatry 56:510–515
Stipursky J, Romao L, Tortelli V, Neto VM, Gomes FC (2011) Neuron-glia signaling: Implications for astrocyte differentiation and synapse formation. Life Sci 89:524–531. doi:10.1016/j.lfs.2011.04.005
Sullivan P (2011) Don’t give up on GWAS Molecular psychiatry
Swanson J et al (2000) Attention deficit/hyperactivity disorder children with a 7-repeat allele of the dopamine receptor D4 gene have extreme behavior but normal performance on critical neuropsychological tests of attention. Proc Natl Acad Sci 97:4754–4759
Taerk E et al (2004) Catechol-O-methyltransferase (COMT) Val108/158 Met polymorphism does not modulate executive function in children with ADHD. BMC Med Genet 5:30
Takahashi N, Sakurai T, Davis KL, Buxbaum JD (2011) Linking oligodendrocyte and myelin dysfunction to neurocircuitry abnormalities in schizophrenia. Prog Neurobiol 93:13–24. doi:10.1016/j.pneurobio.2010.09.004
Tian T, Qin W, Liu B, Wang D, Wang J, Jiang T, Yu C (2013) Catechol-O-methyltransferase Val158Met polymorphism modulates gray matter volume and functional connectivity of the default mode network. PloS One 8:e78697
Tsai S-J, Yu YW-Y, Chen T-J, Chen J-Y, Liou Y-J, Chen M-C, Hong C-J (2003) Association study of a functional catechol-O-methyltransferase-gene polymorphism and cognitive function in healthy females. Neurosci Lett 338:123–126
Tye C, Asherson P, Ashwood KL, Azadi B, Bolton P, McLoughlin G (2014) Attention and inhibition in children with ASD, ADHD and co-morbid ASD + ADHD: an event-related potential study. Psychol Med 44:1101–1116
Valko L et al (2009) Differences in neurophysiological markers of inhibitory and temporal processing deficits in children and adults with ADHD Federation of. Eur Psychophys Ser 23:212–223
van Leeuwen TH et al (1998) The continuous performance test revisited with neuroelectric mapping: impaired orienting in children with attention deficits. BehavBrain Res 94:97–110
Van Rooij D et al (2015) Influence of DAT1 and COMT variants on neural activation during response inhibition in adolescents with attention-deficit/hyperactivity disorder and healthy controls. Psychol Med 45:3159–3170
Vogel C, Laucht M, Furtado E, Becker K, Schmidt M (2006) Association of DRD4 exon III polymorphism with auditory P300 amplitude in 8-year-old children. J Neural Trans 113:1935–1941
Wang S, Yang Y, Xing W, Chen J, Liu C, Luo X (2013) Altered neural circuits related to sustained attention and executive control in children with ADHD: an event-related fMRI. Study Clin Neurophysiol 124:2181–2190
Weinberger DR et al (2001) Prefrontal neurons and the genetics of schizophrenia. Biol Psychiatry 50:825–844
Weiss EM et al (2014) Influences of COMT and 5-HTTLPR polymorphisms on cognitive flexibility in healthy women: inhibition of prepotent responses and memory updating. PloS One 9:e85506
Whiteford HA et al (2013) Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet 382:1575–1586
Winterer G, Egan MF, Kolachana BS, Goldberg TE, Coppola R, Weinberger DR (2006) Prefrontal electrophysiologic “noise” and catechol-O-methyltransferase genotype in schizophrenia. Biol Psychiatry 60:578–584
Wishart HA et al (2011) COMT Val158Met genotype and individual differences in executive function in healthy adults. J Int Neuropsychol Soc 17:174–180
Wong AH, Van Tol HH (2003) Schizophrenia: from phenomenology to neurobiology. Neurosci Biobehav Rev 27:269–306 pii]
Wynn JK, Sugar C, Horan WP, Kern R, Green MF (2010) Mismatch negativity, social cognition, and functioning in schizophrenia patients. Biol Psychiatry 67:940–947. doi:10.1016/j.biopsych.2009.11.024
Yantis S, Schwarzbach J, Serences JT, Carlson RL, Steinmetz MA, Pekar JJ, Courtney SM (2002) Transient neural activity in human parietal cortex during spatial attention shifts. Nat Neurosci 5:995–1002
Yoon JH, Minzenberg MJ, Ursu S, Ryan Walter BS, Wendelken C, Ragland JD, Carter CS (2008) Association of dorsolateral prefrontal cortex dysfunction with disrupted coordinated brain activity in schizophrenia: relationship with impaired cognition, behavioral disorganization, and global function. Am J Psychiatry 165:1006–1014. doi:10.1176/appi.ajp.2008.07060945
Funding
Dr. McLoughlin was supported for this work by the Royal Society and an MRC New Investigator Research Grant (MR/N013182/1). The participation of Drs. Makeig, Palmer and McLoughlin was supported by a gift from The Swartz Foundation (Old Field, NY).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Appendix
Appendix
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
McLoughlin, G., Palmer, J., Makeig, S. et al. EEG Source Imaging Indices of Cognitive Control Show Associations with Dopamine System Genes. Brain Topogr 31, 392–406 (2018). https://doi.org/10.1007/s10548-017-0601-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10548-017-0601-z