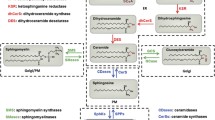
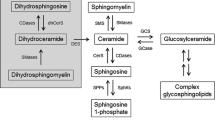
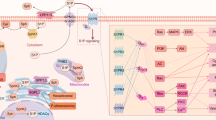
Abstract
Autophagy, the main intracellular process of cytoplasmic material degradation, is involved in cell survival and death. Autophagy is regulated at various levels and novel modulators of its function are being continuously identified. An intriguing recent observation is that among these modulators is the sphingolipid metabolising enzyme, Acid Sphingomyelinase (A-SMase), already known to play a fundamental role in apoptotic cell death participating in several pathophysiological conditions. In this review we analyse and discuss the relationship between autophagy and A-SMase describing how A-SMase may regulate it and defining, for the first time, the existence of an A-SMase-autophagy axis. The imbalance of this axis plays a role in cancer, nervous system, cardiovascular, and hepatic disorders.
Similar content being viewed by others
References
Perrotta C, Clementi E (2010) Biological roles of Acid and neutral sphingomyelinases and their regulation by nitric oxide. Physiology (Bethesda) 25:64–71
Marchesini N, Osta W, Bielawski J, Luberto C, Obeid LM, Hannun YA (2004) Role for mammalian neutral sphingomyelinase 2 in confluence-induced growth arrest of MCF7 cells. J Biol Chem 279:25101–25111
Bartke N, Hannun YA (2009) Bioactive sphingolipids: metabolism and function. J Lipid Res 50(Suppl):S91–S96
Hannun YA, Obeid LM (2002) The Ceramide-centric universe of lipid-mediated cell regulation: stress encounters of the lipid kind. J Biol Chem 277:25847–25850
Hannun YA, Obeid LM (2011) Many ceramides. J Biol Chem 286:27855–27862
Gatt S (1963) Enzymic hydrolysis and synthesis of ceramides. J Biol Chem 238:3131–3133
Horinouchi K, Erlich S, Perl DP, Ferlinz K, Bisgaier CL, Sandhoff K, Desnick RJ, Stewart CL, Schuchman EH (1995) Acid sphingomyelinase deficient mice: a model of types A and B Niemann-Pick disease. Nat Genet 10:288–293
Quintern LE, Schuchman EH, Levran O, Suchi M, Ferlinz K, Reinke H, Sandhoff K, Desnick RJ (1989) Isolation of cDNA clones encoding human acid sphingomyelinase: occurrence of alternatively processed transcripts. EMBO J 8:2469–2473
Hurwitz R, Ferlinz K, Vielhaber G, Moczall H, Sandhoff K (1994) Processing of human acid sphingomyelinase in normal and I-cell fibroblasts. J Biol Chem 269:5440–5445
Ferlinz K, Hurwitz R, Vielhaber G, Suzuki K, Sandhoff K (1994) Occurrence of two molecular forms of human acid sphingomyelinase. Biochem J 301:855–862
Perrotta C, Bizzozero L, Cazzato D, Morlacchi S, Assi E, Simbari F, Zhang Y, Gulbins E, Bassi MT, Rosa P, Clementi E (2010) Syntaxin 4 is required for acid sphingomyelinase activity and apoptotic function. J Biol Chem 285:40240–40251
Ferlinz K, Hurwitz R, Moczall H, Lansmann S, Schuchman EH, Sandhoff K (1997) Functional characterization of the N-glycosylation sites of human acid sphingomyelinase by site-directed mutagenesis. Eur J Biochem 243:511–517
Schuchman EH, Levran O, Pereira LV, Desnick RJ (1992) Structural organization and complete nucleotide sequence of the gene encoding human acid sphingomyelinase (SMPD1). Genomics 12:197–205
Schissel SL, Keesler GA, Schuchman EH, Williams KJ, Tabas I (1998) The cellular trafficking and zinc dependence of secretory and lysosomal sphingomyelinase, two products of the acid sphingomyelinase gene. J Biol Chem 273:18250–18259
Barsacchi R, Perrotta C, Sestili P, Cantoni O, Moncada S, Clementi E (2002) Cyclic GMP-dependent inhibition of acid sphingomyelinase by nitric oxide: an early step in protection against apoptosis. Cell Death Differ 9:1248–1255
Dumitru CA, Gulbins E (2006) TRAIL activates acid sphingomyelinase via a redox mechanism and releases ceramide to trigger apoptosis. Oncogene 25:5612–5625
Zeidan YH, Wu BX, Jenkins RW, Obeid LM, Hannun YA (2008) A novel role for protein kinase Cdelta-mediated phosphorylation of acid sphingomyelinase in UV light-induced mitochondrial injury. FASEB J 22:183–193
Garcia-Barros M, Paris F, Cordon-Cardo C, Lyden D, Rafii S, Haimovitz-Friedman A, Fuks Z, Kolesnick R (2003) Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science 300:1155–1159
Perrotta C, Bizzozero L, Falcone S, Rovere-Querini P, Prinetti A, Schuchman EH, Sonnino S, Manfredi AA, Clementi E (2007) Nitric oxide boosts chemoimmunotherapy via inhibition of acid sphingomyelinase in a mouse model of melanoma. Cancer Res 67:7559–7564
Perrotta C, De Palma C, Falcone S, Sciorati C, Clementi E (2005) Nitric oxide, ceramide and sphingomyelinase-coupled receptors: a tale of enzymes and messengers coordinating cell death, survival and differentiation. Life Sci 77:1732–1739
Prinetti A, Millimaggi D, D’Ascenzo S, Clarkson M, Bettiga A, Chigorno V, Sonnino S, Pavan A, Dolo V (2006) Lack of ceramide generation and altered sphingolipid composition are associated with drug resistance in human ovarian carcinoma cells. Biochem J 395:311–318
Lovat PE, Corazzari M, Goranov B, Piacentini M, Redfern CP (2004) Molecular mechanisms of fenretinide-induced apoptosis of neuroblastoma cells. Ann N Y Acad Sci 1028:81–89
Grassme H, Gulbins E, Brenner B, Ferlinz K, Sandhoff K, Harzer K, Lang F, Meyer TF (1997) Acidic sphingomyelinase mediates entry of N. gonorrhoeae into nonphagocytic cells. Cell 91:605–615
Simonis A, Hebling S, Gulbins E, Schneider-Schaulies S, Schubert-Unkmeir A (2014) Differential activation of acid sphingomyelinase and ceramide release determines invasiveness of Neisseria meningitidis into brain endothelial cells. PLoS Pathog 10:e1004160
Zhang Y, Li X, Carpinteiro A, Gulbins E (2008) Acid sphingomyelinase amplifies redox signaling in Pseudomonas aeruginosa-induced macrophage apoptosis. J Immunol 181:4247–4254
Miller ME, Adhikary S, Kolokoltsov AA, Davey RA (2012) Ebolavirus requires acid sphingomyelinase activity and plasma membrane sphingomyelin for infection. J Virol 86:7473–7483
Grassme H, Riehle A, Wilker B, Gulbins E (2005) Rhinoviruses infect human epithelial cells via ceramide-enriched membrane platforms. J Biol Chem 280:26256–26262
Esen M, Schreiner B, Jendrossek V, Lang F, Fassbender K, Grassme H, Gulbins E (2001) Mechanisms of Staphylococcus aureus induced apoptosis of human endothelial cells. Apoptosis 6:431–439
Gulbins E, Kolesnick R (2003) Raft ceramide in molecular medicine. Oncogene 22:7070–7077
Lacour S, Hammann A, Grazide S, Lagadic-Gossmann D, Athias A, Sergent O, Laurent G, Gambert P, Solary E, Dimanche-Boitrel MT (2004) Cisplatin-induced CD95 redistribution into membrane lipid rafts of HT29 human colon cancer cells. Cancer Res 64:3593–3598
Mollinedo F, Gajate C (2006) Fas/CD95 death receptor and lipid rafts: new targets for apoptosis-directed cancer therapy. Drug Resist Updat 9:51–73
Tchikov V, Bertsch U, Fritsch J, Edelmann B, Schutze S (2011) Subcellular compartmentalization of TNF receptor-1 and CD95 signaling pathways. Eur J Cell Biol 90:467–475
Edelmann B, Bertsch U, Tchikov V, Winoto-Morbach S, Perrotta C, Jakob M, Adam-Klages S, Kabelitz D, Schutze S (2011) Caspase-8 and caspase-7 sequentially mediate proteolytic activation of acid sphingomyelinase in TNF-R1 receptosomes. EMBO J 30:379–394
Algeciras-Schimnich A, Shen L, Barnhart BC, Murmann AE, Burkhardt JK, Peter ME (2002) Molecular ordering of the initial signaling events of CD95. Mol Cell Biol 22:207–220
Herz J, Pardo J, Kashkar H, Schramm M, Kuzmenkina E, Bos E, Wiegmann K, Wallich R, Peters PJ, Herzig S, Schmelzer E, Kronke M, Simon MM, Utermohlen O (2009) Acid sphingomyelinase is a key regulator of cytotoxic granule secretion by primary T lymphocytes. Nat Immunol 10:761–768
Bianco F, Perrotta C, Novellino L, Francolini M, Riganti L, Menna E, Saglietti L, Schuchman EH, Furlan R, Clementi E, Matteoli M, Verderio C (2009) Acid sphingomyelinase activity triggers microparticle release from glial cells. EMBO J 28:1043–1054
Munzer P, Borst O, Walker B, Schmid E, Feijge MA, Cosemans JM, Chatterjee M, Schmidt EM, Schmidt S, Towhid ST, Leibrock C, Elvers M, Schaller M, Seizer P, Ferlinz K, May AE, Gulbins E, Heemskerk JW, Gawaz M, Lang F (2014) Acid sphingomyelinase regulates platelet cell membrane scrambling, secretion, and thrombus formation. Arterioscler Thromb Vasc Biol 34:61–71
Suzuki K, Kubota Y, Sekito T, Ohsumi Y (2007) Hierarchy of Atg proteins in pre-autophagosomal structure organization. Genes Cells 12:209–218
Jin M, Klionsky DJ (2014) Regulation of autophagy: modulation of the size and number of autophagosomes. FEBS Lett 588:2457–2463
Galluzzi L, Pietrocola F, Levine B, Kroemer G (2014) Metabolic control of autophagy. Cell 159:1263–1276
Mizushima N (2007) Autophagy: process and function. Genes Dev 21:2861–2873
Vinod V, Padmakrishnan CJ, Vijayan B, Gopala S (2014) ‘How can I halt thee?’ The puzzles involved in autophagic inhibition. Pharmacol Res 82:1–8
Kenific CM, Debnath J (2015) Cellular and metabolic functions for autophagy in cancer cells. Trends Cell Biol 25:37–45
Banerjee R, Beal MF, Thomas B (2010) Autophagy in neurodegenerative disorders: pathogenic roles and therapeutic implications. Trends Neurosci 33:541–549
Ghavami S, Shojaei S, Yeganeh B, Ande SR, Jangamreddy JR, Mehrpour M, Christoffersson J, Chaabane W, Moghadam AR, Kashani HH, Hashemi M, Owji AA, Los MJ (2014) Autophagy and apoptosis dysfunction in neurodegenerative disorders. Prog Neurobiol 112:24–49
Kesidou E, Lagoudaki R, Touloumi O, Poulatsidou KN, Simeonidou C (2013) Autophagy and neurodegenerative disorders. Neural Regen Res 8:2275–2283
Sandri M, Coletto L, Grumati P, Bonaldo P (2013) Misregulation of autophagy and protein degradation systems in myopathies and muscular dystrophies. J Cell Sci 126:5325–5333
De Palma C, Perrotta A, Pellegrino P, Clementi E, Cervia D (2014) Skeletal muscle homeostasis in Duchenne muscular dystrophy: modulating autophagy as a promising therapeutic strategy. Front Aging Neurosci 6:188
De Palma C, Morisi F, Pambianco S, Assi E, Touvier T, Russo S, Perrotta C, Romanello V, Carnio S, Cappello V, Pellegrino P, Moscheni C, Bassi MT, Sandri M, Cervia D, Clementi E (2014) Deficient nitric oxide signalling impairs skeletal muscle growth and performance: involvement of mitochondrial dysregulation. Skelet Muscle 4:22
De Palma C, Morisi F, Cheli S, Pambianco S, Cappello V, Vezzoli M, Rovere-Querini P, Moggio M, Ripolone M, Francolini M, Sandri M, Clementi E (2012) Autophagy as a new therapeutic target in Duchenne muscular dystrophy. Cell Death Dis 3:e418
Tan CC, Yu JT, Tan MS, Jiang T, Zhu XC, Tan L (2014) Autophagy in aging and neurodegenerative diseases: implications for pathogenesis and therapy. Neurobiol Aging 35:941–957
Jiang T, Yu JT, Zhu XC, Tan MS, Wang HF, Cao L, Zhang QQ, Shi JQ, Gao L, Qin H, Zhang YD, Tan L (2014) Temsirolimus promotes autophagic clearance of amyloid-beta and provides protective effects in cellular and animal models of Alzheimer’s disease. Pharmacol Res 81:54–63
Schweichel JU, Merker HJ (1973) The morphology of various types of cell death in prenatal tissues. Teratology 7:253–266
Shen HM, Codogno P (2011) Autophagic cell death: Loch Ness monster or endangered species? Autophagy 7:457–465
Yu SW, Baek SH, Brennan RT, Bradley CJ, Park SK, Lee YS, Jun EJ, Lookingland KJ, Kim EK, Lee H, Goudreau JL, Kim SW (2008) Autophagic death of adult hippocampal neural stem cells following insulin withdrawal. Stem Cells 26:2602–2610
Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, Mizushima N (2006) Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 441:885–889
Komatsu M, Wang QJ, Holstein GR, Friedrich VL Jr, Iwata J, Kominami E, Chait BT, Tanaka K, Yue Z (2007) Essential role for autophagy protein Atg7 in the maintenance of axonal homeostasis and the prevention of axonal degeneration. Proc Natl Acad Sci USA 104:14489–14494
Geng Y, Kohli L, Klocke BJ, Roth KA (2010) Chloroquine-induced autophagic vacuole accumulation and cell death in glioma cells is p53 independent. Neuro Oncol 12:473–481
Zhao Y, Yang J, Liao W, Liu X, Zhang H, Wang S, Wang D, Feng J, Yu L, Zhu WG (2010) Cytosolic FoxO1 is essential for the induction of autophagy and tumour suppressor activity. Nat Cell Biol 12:665–675
Lavieu G, Scarlatti F, Sala G, Levade T, Ghidoni R, Botti J, Codogno P (2007) Is autophagy the key mechanism by which the sphingolipid rheostat controls the cell fate decision? Autophagy 3:45–47
Cervia D, Perrotta C, Moscheni C, De Palma C, Clementi E (2013) Nitric oxide and sphingolipids control apoptosis and autophagy with a significant impact on Alzheimer’s disease. J Biol Regul Homeost Agents 27:11–22
Young MM, Kester M, Wang HG (2013) Sphingolipids: regulators of crosstalk between apoptosis and autophagy. J Lipid Res 54:5–19
Li Y, Li S, Qin X, Hou W, Dong H, Yao L, Xiong L (2014) The pleiotropic roles of sphingolipid signaling in autophagy. Cell Death Dis 5:e1245
Garcia-Ruiz C, Mato JM, Vance D, Kaplowitz N, Fernandez-Checa JC (2015) Acid sphingomyelinase-ceramide system in steatohepatitis: a novel target regulating multiple pathways. J Hepatol 62:219–233
De Palma C, Perrotta C (2012) Ceramide as a target of chemotherapy: its role in apoptosis and autophagy. Clin Lipidol 7:111–119
Jiang W, Ogretmen B (2014) Autophagy paradox and ceramide. Biochim Biophys Acta 1841:783–792
Vollrath JT, Sechi A, Dreser A, Katona I, Wiemuth D, Vervoorts J, Dohmen M, Chandrasekar A, Prause J, Brauers E, Jesse CM, Weis J, Goswami A (2014) Loss of function of the ALS protein SigR1 leads to ER pathology associated with defective autophagy and lipid raft disturbances. Cell Death Dis 5:e1290
Taniguchi M, Kitatani K, Kondo T, Hashimoto-Nishimura M, Asano S, Hayashi A, Mitsutake S, Igarashi Y, Umehara H, Takeya H, Kigawa J, Okazaki T (2012) Regulation of autophagy and its associated cell death by “sphingolipid rheostat”: reciprocal role of ceramide and sphingosine 1-phosphate in the mammalian target of rapamycin pathway. J Biol Chem 287:39898–39910
Lavieu G, Scarlatti F, Sala G, Carpentier S, Levade T, Ghidoni R, Botti J, Codogno P (2006) Regulation of autophagy by sphingosine kinase 1 and its role in cell survival during nutrient starvation. J Biol Chem 281:8518–8527
Hwang J, Lee S, Lee JT, Kwon TK, Kim DR, Kim H, Park HC, Suk K (2010) Gangliosides induce autophagic cell death in astrocytes. Br J Pharmacol 159:586–603
Hwang J, Lee HJ, Lee WH, Suk K (2010) NF-kappaB as a common signaling pathway in ganglioside-induced autophagic cell death and activation of astrocytes. J Neuroimmunol 226:66–72
Matarrese P, Garofalo T, Manganelli V, Gambardella L, Marconi M, Grasso M, Tinari A, Misasi R, Malorni W, Sorice M (2014) Evidence for the involvement of GD3 ganglioside in autophagosome formation and maturation. Autophagy 10:750–765
Smith EL, Schuchman EH (2008) Acid sphingomyelinase overexpression enhances the antineoplastic effects of irradiation in vitro and in vivo. Mol Ther 16:1565–1571
Patschan S, Chen J, Polotskaia A, Mendelev N, Cheng J, Patschan D, Goligorsky MS (2008) Lipid mediators of autophagy in stress-induced premature senescence of endothelial cells. Am J Physiol Heart Circ Physiol 294:H1119–H1129
Lee JK, Jin HK, Park MH, Kim BR, Lee PH, Nakauchi H, Carter JE, He X, Schuchman EH, Bae JS (2014) Acid sphingomyelinase modulates the autophagic process by controlling lysosomal biogenesis in Alzheimer’s disease. J Exp Med 211:1551–1570
Toops KA, Tan LX, Jiang Z, Radu RA, Lakkaraju A (2015) Cholesterol-mediated activation of acid sphingomyelinase disrupts autophagy in the retinal pigment epithelium. Mol Biol Cell 26:1–14
Gabande-Rodriguez E, Boya P, Labrador V, Dotti CG, Ledesma MD (2014) High sphingomyelin levels induce lysosomal damage and autophagy dysfunction in Niemann Pick disease type A. Cell Death Differ 21:864–875
Fucho R, Martinez L, Baulies A, Torres S, Tarrats N, Fernandez A, Ribas V, Astudillo AM, Balsinde J, Garcia-Roves P, Elena M, Bergheim I, Lotersztajn S, Trautwein C, Appelqvist H, Paton AW, Paton JC, Czaja MJ, Kaplowitz N, Fernandez-Checa JC, Garcia-Ruiz C (2014) ASMase regulates autophagy and lysosomal membrane permeabilization and its inhibition prevents early stage non-alcoholic steatohepatitis. J Hepatol 61(5):1126–1134
Li X, Xu M, Pitzer AL, Xia M, Boini KM, Li PL, Zhang Y (2014) Control of autophagy maturation by acid sphingomyelinase in mouse coronary arterial smooth muscle cells: protective role in atherosclerosis. J Mol Med 92:473–485
Petersen NH, Olsen OD, Groth-Pedersen L, Ellegaard AM, Bilgin M, Redmer S, Ostenfeld MS, Ulanet D, Dovmark TH, Lonborg A, Vindelov SD, Hanahan D, Arenz C, Ejsing CS, Kirkegaard T, Rohde M, Nylandsted J, Jaattela M (2013) Transformation-associated changes in sphingolipid metabolism sensitize cells to lysosomal cell death induced by inhibitors of acid sphingomyelinase. Cancer Cell 24:379–393
Ostenfeld MS, Hoyer-Hansen M, Bastholm L, Fehrenbacher N, Olsen OD, Groth-Pedersen L, Puustinen P, Kirkegaard-Sorensen T, Nylandsted J, Farkas T, Jaattela M (2008) Anti-cancer agent siramesine is a lysosomotropic detergent that induces cytoprotective autophagosome accumulation. Autophagy 4:487–499
Rossi M, Munarriz ER, Bartesaghi S, Milanese M, Dinsdale D, Guerra-Martin MA, Bampton ET, Glynn P, Bonanno G, Knight RA, Nicotera P, Melino G (2009) Desmethylclomipramine induces the accumulation of autophagy markers by blocking autophagic flux. J Cell Sci 122:3330–3339
Russ DW, Wills AM, Boyd IM, Krause J (2014) Weakness, SR function and stress in gastrocnemius muscles of aged male rats. Exp Gerontol 50:40–44
Russ DW, Krause J, Wills A, Arreguin R (2012) “SR stress” in mixed hindlimb muscles of aging male rats. Biogerontology 13:547–555
Wohlgemuth SE, Seo AY, Marzetti E, Lees HA, Leeuwenburgh C (2010) Skeletal muscle autophagy and apoptosis during aging: effects of calorie restriction and life-long exercise. Exp Gerontol 45:138–148
Sui X, Chen R, Wang Z, Huang Z, Kong N, Zhang M, Han W, Lou F, Yang J, Zhang Q, Wang X, He C, Pan H (2013) Autophagy and chemotherapy resistance: a promising therapeutic target for cancer treatment. Cell Death Dis 4:e838
Furuya H, Shimizu Y, Kawamori T (2011) Sphingolipids in cancer. Cancer Metastasis Rev 30:567–576
Bizzozero L, Cazzato D, Cervia D, Assi E, Simbari F, Pagni F, De Palma C, Monno A, Verdelli C, Querini PR, Russo V, Clementi E, Perrotta C (2014) Acid sphingomyelinase determines melanoma progression and metastatic behaviour via the microphtalmia-associated transcription factor signalling pathway. Cell Death Differ 21:507–520
Bionda C, Hadchity E, Alphonse G, Chapet O, Rousson R, Rodriguez-Lafrasse C, Ardail D (2007) Radioresistance of human carcinoma cells is correlated to a defect in raft membrane clustering. Free Radic Biol Med 43:681–694
Haughey NJ (2010) Sphingolipids in neurodegeneration. Neuromolecular Med 12:301–305
Haughey NJ, Bandaru VV, Bae M, Mattson MP (2010) Roles for dysfunctional sphingolipid metabolism in Alzheimer’s disease neuropathogenesis. Biochim Biophys Acta 1801:878–886
Assi E, Cazzato D, De Palma C, Perrotta C, Clementi E, Cervia D (2013) Sphingolipids and brain resident macrophages in neuroinflammation: an emerging aspect of nervous system pathology. Clin Dev Immunol 2013:309302
He X, Huang Y, Li B, Gong CX, Schuchman EH (2010) Deregulation of sphingolipid metabolism in Alzheimer’s disease. Neurobiol Aging 31:398–408
Muhle C, Amova V, Biermann T, Bayerlein K, Richter-Schmidinger T, Kraus T, Reichel M, Gulbins E, Kornhuber J (2014) Sex-dependent decrease of sphingomyelinase activity during alcohol withdrawal treatment. Cell Physiol Biochem 34:71–81
Gulbins E, Palmada M, Reichel M, Luth A, Bohmer C, Amato D, Muller CP, Tischbirek CH, Groemer TW, Tabatabai G, Becker KA, Tripal P, Staedtler S, Ackermann TF, van Brederode J, Alzheimer C, Weller M, Lang UE, Kleuser B, Grassme H, Kornhuber J (2013) Acid sphingomyelinase-ceramide system mediates effects of antidepressant drugs. Nat Med 19:934–938
Reichel M, Greiner E, Richter-Schmidinger T, Yedibela O, Tripal P, Jacobi A, Bleich S, Gulbins E, Kornhuber J (2010) Increased acid sphingomyelinase activity in peripheral blood cells of acutely intoxicated patients with alcohol dependence. Alcohol Clin Exp Res 34:46–50
Reichel M, Beck J, Muhle C, Rotter A, Bleich S, Gulbins E, Kornhuber J (2011) Activity of secretory sphingomyelinase is increased in plasma of alcohol-dependent patients. Alcohol Clin Exp Res 35:1852–1859
Lista P, Straface E, Brunelleschi S, Franconi F, Malorni W (2011) On the role of autophagy in human diseases: a gender perspective. J Cell Mol Med 15:1443–1457
Schissel SL, Jiang X, Tweedie-Hardman J, Jeong T, Camejo EH, Najib J, Rapp JH, Williams KJ, Tabas I (1998) Secretory sphingomyelinase, a product of the acid sphingomyelinase gene, can hydrolyze atherogenic lipoproteins at neutral pH. Implications for atherosclerotic lesion development. J Biol Chem 273:2738–2746
Leger AJ, Mosquea LM, Li L, Chuang W, Pacheco J, Taylor K, Luo Z, Piepenhagen P, Ziegler R, Moreland R, Urabe A, Jiang C, Cheng SH, Yew NS (2011) Adeno-associated virus-mediated expression of acid sphingomyelinase decreases atherosclerotic lesion formation in apolipoprotein E(-/-) mice. J Gene Med 13:324–332
Devlin CM, Leventhal AR, Kuriakose G, Schuchman EH, Williams KJ, Tabas I (2008) Acid sphingomyelinase promotes lipoprotein retention within early atheromata and accelerates lesion progression. Arterioscler Thromb Vasc Biol 28:1723–1730
Oorni K, Posio P, Ala-Korpela M, Jauhiainen M, Kovanen PT (2005) Sphingomyelinase induces aggregation and fusion of small very low-density lipoprotein and intermediate-density lipoprotein particles and increases their retention to human arterial proteoglycans. Arterioscler Thromb Vasc Biol 25:1678–1683
Deaciuc IV, Nikolova-Karakashian M, Fortunato F, Lee EY, Hill DB, McClain CJ (2000) Apoptosis and dysregulated ceramide metabolism in a murine model of alcohol-enhanced lipopolysaccharide hepatotoxicity. Alcohol Clin Exp Res 24:1557–1565
Liangpunsakul S, Rahmini Y, Ross RA, Zhao Z, Xu Y, Crabb DW (2012) Imipramine blocks ethanol-induced ASMase activation, ceramide generation, and PP2A activation, and ameliorates hepatic steatosis in ethanol-fed mice. Am J Physiol Gastrointest Liver Physiol 302:G515–G523
Samad F, Hester KD, Yang G, Hannun YA, Bielawski J (2006) Altered adipose and plasma sphingolipid metabolism in obesity: a potential mechanism for cardiovascular and metabolic risk. Diabetes 55:2579–2587
Moles A, Tarrats N, Morales A, Dominguez M, Bataller R, Caballeria J, Garcia-Ruiz C, Fernandez-Checa JC, Mari M (2010) Acidic sphingomyelinase controls hepatic stellate cell activation and in vivo liver fibrogenesis. Am J Pathol 177:1214–1224
Smith EL, Schuchman EH (2008) The unexpected role of acid sphingomyelinase in cell death and the pathophysiology of common diseases. Faseb J 22:3419–3431
Acknowledgments
This work was supported by “Ministero della Salute, Giovani Ricercatori 2011–2012” Grant to C.D.P. and “Ricerca corrente 2015” Grant to E.C.; “Ministero dell’Istruzione, Università e Ricerca, PRIN2010-2011” Grants to E.C. and D.C.; “Università di Milano, Giovani Ricercatori-Linea B” Grant to C.P.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Perrotta, C., Cervia, D., De Palma, C. et al. The emerging role of Acid Sphingomyelinase in autophagy. Apoptosis 20, 635–644 (2015). https://doi.org/10.1007/s10495-015-1101-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-015-1101-9