Abstract
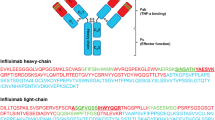
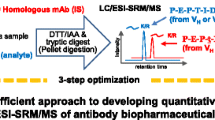
Liquid chromatography tandem mass spectrometry (LC–MS/MS), especially triple-quadrupole mass spectrometry, has become an attractive alternative method to ligand binding assays for therapeutic monoclonal antibody quantification in biological samples, but the use of an internal standard with infliximab LC–MS/MS assays has not been reported yet. In this study, an improved LC–MS/MS method for quantification of infliximab in human serum was developed and validated. A surrogate peptide was used as a representative of infliximab which was cleaved for the quantification of infliximab based on LC–MS/MS assay. A stable isotope-labeled signature peptide was used as the internal standard (IS). The results showed linearity in the range of 0.39–100 μg mL−1; the lower limit of quantification (LLOQ), and the lower limit of detection were 0.39 and 0.0975 μg mL−1, respectively. The quality control (QC) data showed that the within-run, between-run precision (%RSD) and accuracy (%RE) conformed to the acceptance criteria of ±15 % for calibration standards and QCs (±20 % at the LLOQ). Other validation parameters including selectivity, methanol precipitation efficiency, serum matrix effect, stability, and auto-sampler carry-over were also evaluated. This improved LC–MS/MS method might be a promising LC–MS-based methodology for pharmacokinetic studies of other recombinant monoclonal antibodies.





Similar content being viewed by others
References
Balkwill F (2009) Nat Rev Cancer 9:361–371
Elliott MJ, Maini R, Feldmann M, Long-Fox A, Charles P, Bijl J, Woody J (1994) Lancet 344:1125–1127
Van Dullemen H, Van Deventer S, Hommes D, Bijl H, Jansen J, Tytgat G (1995) Gastroenterology 109:129–135
Knight DM, Trinh H, Le J, Siegel S, Shealy D, McDonough M, Scallon B, Moore MA, Vilcek J, Daddona P (1993) Mol Immunol 30:1443–1453
Martínez-Borra J, López-Larrea C, González S, Fuentes D, Dieguez A, Deschamps EM, Perez-Pariente J, López-Vázquez A, de Francisco R, Rodrigo L (2002) Am J Gastroenterol 97:2350–2356
Targan SR, Hanauer SB, van Deventer SJ, Mayer L, Present DH, Braakman T, DeWoody KL, Schaible TF, Rutgeerts PJ (1997) New Engl J Med 337:1029–1036
Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, Rachmilewitz D, Wolf DC, Olson A, Bao W (2002) Lancet 359:1541–1549
Present DH, Rutgeerts P, Targan S, Hanauer SB, Mayer L, Van Hogezand R, Podolsky DK, Sands BE, Braakman T, DeWoody KL (1999) New Engl J Med 340:1398–1405
Chey WY (2001) Inflamm Bowel Dis 7:S30–S33
Järnerot G, Hertervig E, Friis-Liby I, Blomquist L, Karlén P, Grännö C, Vilien M, Ström M, Danielsson Å, Verbaan H (2005) Gastroenterology 128:1805–1811
Maini SR (2004) Rheum Dis Clin N Am 30:329–347
Braun J, Brandt J, Listing J, Zink A, Alten R, Golder W, Gromnica-lhle E, Kellner H, Krause A, Schneider M (2002) Lancet 359:1187–1193
Chaudhari U, Romano P, Mulcahy L, Dooley L, Baker D, Gottlieb A (2001) Lancet 357:1842–1847
Zippi M, Cassieri C, Avallone EV, Pica R (2013) World J Clin Cases 1:191
Brown E, Charles K, Hoare S, Rye R, Jodrell D, Aird R, Vora R, Prabhakar U, Nakada M, Corringham R (2008) Ann Oncol 19:1340–1346
Gisbert JP, Panés J (2009) Am J Gastroenterol 104:760–767
Miheller P, Kiss LS, Lorinczy K, Lakatos PL (2012) Expert Opin Biol Ther 12:179–192
Regueiro M, Siemanowski B, Kip KE, Plevy S (2007) Inflamm Bowel Dis 13:1093–1099
Mulleman D, Méric J-C, Paintaud G, Ducourau E, Magdelaine-Beuzelin C, Valat J-P, Goupille P, GICC CNdlRSU (2009) Arthritis Res Ther 11(6):R178
Dasgupta A (2008) Introduction to therapeutic drug monitoring. In: Dasgupta A (ed) Handbook of drug monitoring methods. Springer, pp 1–39
Baert F, Noman M, Vermeire S, Van Assche G, D’Haens G, Carbonez A, Rutgeerts P (2003) New Engl J Med 348:601–608
Aarden L, Ruuls SR, Wolbink G (2008) Curr Opin Immunol 20:431–435
Anderson NL, Anderson NG, Haines LR, Hardie DB, Olafson RW, Pearson TW (2004) J Proteome Res 3:235–244
Vande Casteele N, Buurman D, Sturkenboom M, Kleibeuker J, Vermeire S, Rispens T, Kleij D, Gils A, Dijkstra G (2012) Aliment Pharm Therap 36:765–771
Wang S-L, Hauenstein S, Ohrmund L, Shringarpure R, Salbato J, Reddy R, McCowen K, Shah S, Lockton S, Chuang E (2013) J Pharm Biomed Anal 78:39–44
Rawlins ML, Roberts WL (2004) Clin Chem 50:2338–2344
Mongia SK, Rawlins ML, Owen WE, Roberts WL (2006) Am J Clin Pathol 125:921–927
La’ulu SL, Roberts WL (2007) Am J Clin Pathol 127:436–440
Palandra J, Finelli A, Zhu M, Masferrer J, Neubert H (2013) Anal Chem 85:5522–5529
Rotmensch S, Cole LA (2000) Lancet 355:712–715
Morgan BR, Tarter TH (2001) J Urol 166:2311–2312
Ciccimaro E, Blair IA (2010) Bioanalysis 2:311–341
Zhang SW, Jian W (2014) Rev Anal Chem 33:31–47
Becker JO, Hoofnagle AN (2012) Bioanalysis 4:281–290
Hilger M, Bonaldi T, Gnad F, Mann M (2009) Mol Cell Proteomics 8:1908–1920
Dephoure N, Zhou C, Villén J, Beausoleil SA, Bakalarski CE, Elledge SJ, Gygi SP (2008) Proc Natl Acad Sci USA 105:10762–10767
Heudi O, Barteau S, Zimmer D, Schmidt J, Bill K, Lehmann N, Bauer C, Kretz O (2008) Anal Chem 80:4200–4207
Desiderio DM, Kai M (1983) Biol Mass Spectrom 10:471–479
Kennedy JJ, Abbatiello SE, Kim K, Yan P, Whiteaker JR, Lin C, Kim JS, Zhang Y, Wang X, Ivey RG (2013) Nat Methods 11:149–155
Ji C, Sadagopan N, Zhang Y, Lepsy C (2009) Anal Chem 81:9321–9328
Steenholdt C, Bendtzen K, Brynskov J, Ainsworth MA (2014) Am J Gastroenterol 109:1055–1064
Jiang H, Zeng J, Titsch C, Voronin K, Akinsanya B, Luo L, Shen H, Desai DD, Allentoff A , Aubry A-Fo (2013) Anal Chem 85:9859–9867
FDA (2001) Guidance for industry: bioanalytical method validation, pp 1–21
Zimmer D (2014) Bioanalysis 6:13–19
Khanna R, Sattin B, Afif W, Benchimol E, Bernard EJ, Bitton A, Bressler B, Fedorak R, Ghosh S, Greenberg G (2013) Aliment Pharm Therap 38:447–459
Li H, Ortiz R, Tran L, Hall M, Spahr C, Walker K, Laudemann J, Miller S, Salimi-Moosavi H, Lee JW (2012) Anal Chem 84:1267–1273
Brun V, Dupuis A, Adrait A, Marcellin M, Thomas D, Vandenesch F, Garin J (2007) Mol Cell Protemics 6:2139–2149
Beynon RJ, Doherty MK, Pratt JM, Gaskell SJ (2005) Nat Methods 2:587–589
Acknowledgments
We thank Waters Company Laboratories for cooperation with us. This work was supported by grants from the Natural Science Foundation of China (81330061), Ministry of Science and Technology of China (973 projects 2010CB833605 and 863 projects 2014AA021004), State Key Project for New Drug Development (2013ZX09101021; 2013ZX09401303), Shanghai Commission of Science and Technology (Key Laboratory and Projects 13DZ1930100), and Shanghai Excellent technical leader (13XD1424000).
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
X. Peng, B. Liu, Y. Li were contributed equally to this work.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Peng, X., Liu, B., Li, Y. et al. Development and Validation of LC–MS/MS Method for the Quantitation of Infliximab in Human Serum. Chromatographia 78, 521–531 (2015). https://doi.org/10.1007/s10337-015-2866-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-015-2866-2