Abstract
Objectives
We conduct a cost-utility analysis of inotuzumab ozogamicin (INO) versus chemotherapy as the standard of care (SOC) for adults with relapsed or refractory B cell acute lymphoblastic leukemia.
Methods
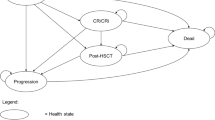
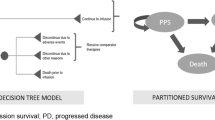
A Markov model incorporating transition probabilities between health states was applied to simulate disease progression. The model inputs, including overall survival, progression-free survival, and utility parameters, were obtained from the INO-VATE ALL trial and literatures. The Taiwan Cancer Registry Database and the Health and Welfare Database were utilized to identify the patient cohort and medical costs from the perspective of National Health Insurance Administration. The lifetime medical costs (in 2017 US dollars), quality-adjusted life years (QALYs) gained, and associated incremental cost-effectiveness ratio (ICER) were the main study outcomes.
Results
The lifetime medical costs for INO and SOC were $176,795 and $69,496, and the QALYs gained were 2.25 and 0.84, respectively. The ICER for INO versus SOC was $76,044 per QALY gained, which is slightly more than three times Taiwan’s gross domestic product per capita (i.e., $73,224). Favorable economic results for INO versus SOC were found with an increased time horizon for model simulation, less discounting for the future benefit, and higher stem cell transplantation (SCT) rate after INO treatment; and among patients aged less than 55 years, with no SCT history, or in the first salvage treatment.
Conclusions
INO versus SOC has higher costs but is more effective. The use of INO is favorable for patients in the early treatment course and when more future benefit associated with INO is considered.



Similar content being viewed by others
References
Terwilliger, T., Abdul-Hay, M.: Acute lymphoblastic leukemia: a comprehensive review and 2017 update. Blood Cancer J. 7(6), e577 (2017). https://doi.org/10.1038/bcj.2017.53
Uy, N., Nadeau, M., Stahl, M., Zeidan, A.M.: Inotuzumab ozogamicin in the treatment of relapsed/refractory acute B cell lymphoblastic leukemia. J. Blood Med. 9, 67–74 (2018). https://doi.org/10.2147/jbm.S136575
Health Promotion Administration Taiwan (2015) Taiwan Cancer Registry Annual Report. https://www.hpa.gov.tw/Pages/Detail.aspx?nodeid=269&pid=8084. Accessed 14 Aug 2018
Kantarjian, H.M., DeAngelo, D.J., Stelljes, M., Martinelli, G., Liedtke, M., Stock, W., Gokbuget, N., O'Brien, S., Wang, K., Wang, T., Paccagnella, M.L., Sleight, B., Vandendries, E., Advani, A.S.: Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N. Engl. J. Med. 375(8), 740–753 (2016). https://doi.org/10.1056/NEJMoa1509277
Hung, M.C., Lai, W.W., Chen, H.H., Lee, J.C., Lin, Y.J., Hsiao, J.R., Cheng, Y.M., Shan, Y.S., Su, W.C., Wang, J.D.: Cost effectiveness of cancer treatment in Taiwan. J. Formos. Med. Assoc. 115(8), 609–618 (2016). https://doi.org/10.1016/j.jfma.2016.04.002
Sanders, G.D., Neumann, P.J., Basu, A., Brock, D.W., Feeny, D., Krahn, M., Kuntz, K.M., Meltzer, D.O., Owens, D.K., Prosser, L.A., Salomon, J.A., Sculpher, M.J., Trikalinos, T.A., Russell, L.B., Siegel, J.E., Ganiats, T.G.: Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA 316(10), 1093–1103 (2016). https://doi.org/10.1001/jama.2016.12195
Gökbuget, N., Stanze, D., Beck, J., Diedrich, H., Horst, H.-A., Hüttmann, A., Kobbe, G., Kreuzer, K.-A., Leimer, L., Reichle, A., Schaich, M., Schwartz, S., Serve, H., Starck, M., Stelljes, M., Stuhlmann, R., Viardot, A., Wendelin, K., Freund, M., Hoelzer, D.: Outcome of relapsed adult lymphoblastic leukemia depends on response to salvage chemotherapy, prognostic factors, and performance of stem cell transplantation. Blood 120(10), 2032–2041 (2012). https://doi.org/10.1182/blood-2011-12-399287
Kantarjian, H., Thomas, D., Jorgensen, J., Jabbour, E., Kebriaei, P., Rytting, M., York, S., Ravandi, F., Kwari, M., Faderl, S., Rios, M.B., Cortes, J., Fayad, L., Tarnai, R., Wang, S.A., Champlin, R., Advani, A., O'Brien, S.: Inotuzumab ozogamicin, an anti-CD22-calecheamicin conjugate, for refractory and relapsed acute lymphocytic leukaemia: a phase 2 study. Lancet Oncol. 13(4), 403–411 (2012). https://doi.org/10.1016/s1470-2045(11)70386-2
Aristides, M., Barlev, A., Barber, B., Gijsen, M., Quinn, C.: Population preference values for health states in relapsed or refractory B-precursor acute lymphoblastic leukemia in the United Kingdom. Health Qual Life Outcomes 13, 181 (2015). https://doi.org/10.1186/s12955-015-0377-3
Latimer, N.R.: NICE Decision Support Unit Technical Support Documents. In: Survival analysis for economic evaluations alongside clinical trials—extrapolation with patient-level data. National Institute for Health and Care Excellence (NICE), London (2013)
Fielding, A.K., Richards, S.M., Chopra, R., Lazarus, H.M., Litzow, M.R., Buck, G., Durrant, I.J., Luger, S.M., Marks, D.I., Franklin, I.M., McMillan, A.K., Tallman, M.S., Rowe, J.M., Goldstone, A.H.: Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood 109(3), 944–950 (2007). https://doi.org/10.1182/blood-2006-05-018192
Tavernier, E., Boiron, J.M., Huguet, F., Bradstock, K., Vey, N., Kovacsovics, T., Delannoy, A., Fegueux, N., Fenaux, P., Stamatoullas, A., Tournilhac, O., Buzyn, A., Reman, O., Charrin, C., Boucheix, C., Gabert, J., Lheritier, V., Vernant, J.P., Dombret, H., Thomas, X.: Outcome of treatment after first relapse in adults with acute lymphoblastic leukemia initially treated by the LALA-94 trial. Leukemia 21(9), 1907–1914 (2007). https://doi.org/10.1038/sj.leu.2404824
Chaidos, A., Kanfer, E., Apperley, J.F.: Risk assessment in haemotopoietic stem cell transplantation: disease and disease stage. Best Pract. Res. Clin. Haematol. 20(2), 125–154 (2007). https://doi.org/10.1016/j.beha.2006.10.003
Martin, P.J., Counts Jr., G.W., Appelbaum, F.R., Lee, S.J., Sanders, J.E., Deeg, H.J., Flowers, M.E., Syrjala, K.L., Hansen, J.A., Storb, R.F., Storer, B.E.: Life expectancy in patients surviving more than 5 years after hematopoietic cell transplantation. J. Clin. Oncol. 28(6), 1011–1016 (2010). https://doi.org/10.1200/jco.2009.25.6693
Taiwan Department of Statistics: Abridged life table in Republic of China. https://www.moi.gov.tw/stat/node.aspx?cate_sn=&belong_sn=6189&sn=6190 (2016). Accessed 19 May 2018
Kurosawa, S., Yamaguchi, T., Mori, T., Kanamori, H., Onishi, Y., Emi, N., Fujisawa, S., Kohno, A., Nakaseko, C., Saito, B., Kondo, T., Hino, M., Nawa, Y., Kato, S., Hashimoto, A., Fukuda, T.: Patient-reported quality of life after allogeneic hematopoietic cell transplantation or chemotherapy for acute leukemia. Bone Marrow Transplant. 50(9), 1241–1249 (2015). https://doi.org/10.1038/bmt.2015.137
Ara, R., Brazier, J.E.: Populating an economic model with health state utility values: moving toward better practice. Value Health 13(5), 509–518 (2010). https://doi.org/10.1111/j.1524-4733.2010.00700.x
Ministry of Health and Welfare Department of Statistics. Organization of Data Science Center (2018). https://dep.mohw.gov.tw/DOS/cp-2499-3563-113.html. Accessed 25 Feb 2019
Kiehl, M.G., Kraut, L., Schwerdtfeger, R., Hertenstein, B., Remberger, M., Kroeger, N., Stelljes, M., Bornhaeuser, M., Martin, H., Scheid, C., Ganser, A., Zander, A.R., Kienast, J., Ehninger, G., Hoelzer, D., Diehl, V., Fauser, A.A., Ringden, O.: Outcome of allogeneic hematopoietic stem-cell transplantation in adult patients with acute lymphoblastic leukemia: no difference in related compared with unrelated transplant in first complete remission. J. Clin. Oncol. 22(14), 2816–2825 (2004). https://doi.org/10.1200/jco.2004.07.130
Ministry of Health and Welfare: Height, weight, and body surface area of residents. https://dep.mohw.gov.tw/DOS/cp-1720-7365-113.html (2016). Accessed 14 Aug 2018
Nadler, E., Eckert, B., Neumann, P.J.: Do oncologists believe new cancer drugs offer good value? Oncologist 11(2), 90–95 (2006). https://doi.org/10.1634/theoncologist.11-2-90
Ubel, P.A., Berry, S.R., Nadler, E., Bell, C.M., Kozminski, M.A., Palmer, J.A., Evans, W.K., Strevel, E.L., Neumann, P.J.: In a survey, marked inconsistency in how oncologists judged value of high-cost cancer drugs in relation to gains in survival. Health Aff. (Millwood) 31(4), 709–717 (2012). https://doi.org/10.1377/hlthaff.2011.0251
Delea, T.E., Amdahl, J., Boyko, D., Hagiwara, M., Zimmerman, Z.F., Franklin, J.L., Cong, Z., Hechmati, G., Stein, A.: Cost-effectiveness of blinatumomab versus salvage chemotherapy in relapsed or refractory Philadelphia-chromosome-negative B-precursor acute lymphoblastic leukemia from a US payer perspective. J. Med. Econ. 20(9), 911–922 (2017). https://doi.org/10.1080/13696998.2017.1344127
Stopeck, A., Rader, M., Henry, D., Danese, M., Halperin, M., Cong, Z., Qian, Y., Dansey, R., Chung, K.: Cost-effectiveness of denosumab vs zoledronic acid for prevention of skeletal-related events in patients with solid tumors and bone metastases in the United States. J. Med. Econ. 15(4), 712–723 (2012). https://doi.org/10.3111/13696998.2012.675380
Ahn, M.J., Tsai, C.M., Hsia, T.C., Wright, E., Chang, J.W., Kim, H.T., Kim, J.H., Kang, J.H., Kim, S.W., Bae, E.J., Kang, M., Lister, J., Walzer, S.: Cost-effectiveness of bevacizumab-based therapy versus cisplatin plus pemetrexed for the first-line treatment of advanced non-squamous NSCLC in Korea and Taiwan. Asia Pac. J. Clin. Oncol. 7(Suppl 2), 22–33 (2011). https://doi.org/10.1111/j.1743-7563.2011.01399.x
Lang, H.C., Chen, H.W., Chiou, T.J., Chan, A.L.: The real-world cost-effectiveness of adjuvant trastuzumab in HER-2/neu-positive early breast cancer in Taiwan. J. Med. Econ. 19(10), 923–927 (2016). https://doi.org/10.1080/13696998.2016.1185013
Leung, H.W.C., Chan, A.L.F., Muo, C.H., Leung, J.H.: Cost-effectiveness of pertuzumab combined with trastuzumab and docetaxel as a first-line treatment for HER-2 positive metastatic breast cancer. Expert Rev. Pharmacoecon. Outcomes Res. 18(2), 207–213 (2018). https://doi.org/10.1080/14737167.2018.1386559
Marks, D.I., Kebriaei, P., Stelljes, M., Gokbuget, N., Kantarjian, H., Advani, A.S., Merchant, A., Stock, W., Cassaday, R.D., Wang, T., Zhang, H., Loberiza, F., Vandendries, E., DeAngelo, D.J.: Outcomes of allogeneic stem cell transplantation after inotuzumab ozogamicin treatment for relapsed or refractory acute lymphoblastic leukemia. Biol. Blood Marrow Transplant. (2019). https://doi.org/10.1016/j.bbmt.2019.04.020
Kantarjian, H.M., DeAngelo, D.J., Stelljes, M., Liedtke, M., Stock, W., Gokbuget, N., O'Brien, S.M., Jabbour, E., Wang, T., Liang White, J., Sleight, B., Vandendries, E., Advani, A.S.: Inotuzumab ozogamicin versus standard of care in relapsed or refractory acute lymphoblastic leukemia: Final report and long-term survival follow-up from the randomized, phase 3 INO-VATE study. Cancer (2019). https://doi.org/10.1002/cncr.32116
Acknowledgements
We gratefully thank National Cheng Kung University and its affiliated hospital for all support. The Excel model was originally developed by Ingress Health.
Funding
This project was supported by grants from the Ministry of Science and Technology in Taiwan (Grant MOST 107-2320-B-006-034) and Pfizer, Inc. (recipient: Huang-Tz Ou). The publication of study results was not contingent on the sponsors’ approval or censorship of the manuscript.
Author information
Authors and Affiliations
Contributions
TYL study design, data collection, model operation, result interpretation, writing and revision of draft and final manuscript. HYC data collection, statistical analysis. TYC, SSL study design, clinical consultation, result interpretation, revision of the manuscript. WTF, YWL clinical consultation, revision of the manuscript. YCW study design, result interpretation, revision of the manuscript. HTO study design, result interpretation, writing and revision of draft and final manuscript. TYL and HTO are the guarantors of this work and had full access to all the data in the study.
Corresponding author
Ethics declarations
Conflict of interest
TYL, HYC, TYC, SSL and HTO have no conflicts of interest. YCW, WTF and YWL are employees of Pfizer.
Ethical approval
This study was approved by the Institutional Review Board of National Cheng Kung University Hospital (A-EX-106-029).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, TY., Chen, HY., Chen, TY. et al. Cost-utility analysis of inotuzumab ozogamicin for relapsed or refractory B cell acute lymphoblastic leukemia from the perspective of Taiwan’s health care system. Eur J Health Econ 21, 1105–1116 (2020). https://doi.org/10.1007/s10198-020-01207-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10198-020-01207-7
Keywords
- Acute lymphoblastic leukemia
- Inotuzumab ozogamicin
- Standard of care
- Cost-effectiveness analysis
- Economic modeling