Abstract
Background
The role of adjuvant chemotherapy has not yet been established for patients with resected biliary tract cancer. S-1 has been shown to exert activity against advanced biliary tract cancer. Therefore, we evaluated the feasibility of adjuvant chemotherapy with S-1 in patients with resected biliary tract cancer.
Methods
Patients with complete macroscopic resection of intrahepatic/extrahepatic bile duct, gall bladder, or ampullary cancer were eligible. S-1 was administered orally twice daily for 4 weeks every 6 weeks, up to 4 cycles. The treatment was continued up to 24 weeks or until recurrence/appearance of unacceptable toxicity. The primary endpoint was the treatment completion rate, which was defined as the percentage of patients who received a relative dose intensity of ≥ 75%. This trial was registered as UMIN000004051.
Results
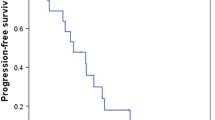
Thirty-three patients were enrolled between June 2010 and March 2011. The relative dose intensity was ≥ 75% in 27 patients representing a treatment completion rate of 81.8%. The most common grade 3/4 adverse event was neutropenia (18%). Grade 2 nausea or diarrhea was observed in 12%. The 3-year relapse-free survival rate was 39.4%. The 3-year survival rate was 54.5%.
Conclusion
Adjuvant chemotherapy with S-1 is feasible treatment in patients with resected biliary tract cancer. It is necessary to conduct a phase III study to confirm the efficacy of adjuvant therapy of S-1 in patients with resected BTC.


Similar content being viewed by others
References
Vital Statistics Japan. Ministry of Health, Labour and Welfare
Ishihara S, Horiguchi A, Miyakawa S et al (2016) Biliary tract cancer registry in Japan from 2008 to 2013. J Hepatobiliary Pancreat Sci 23:149–157
Kudo M, Izumi N, Ichida T et al (2016) Report of the 19th follow-up survey of primary liver cancer in Japan. Hepatol Res 46:372–390
Takada T, Amano H, Yasuda H et al (2002) Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma? A phase III multicenter prospective randomized controlled trial in patients with resected pancreaticobiliary carcinoma. Cancer 95:1685–1695
Neoptolemos JP, Moore MJ, Cox TF et al (2012) Effect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine vs observation on survival in patients with resected periampullary adenocarcinoma: the ESPAC-3 periampullary cancer randomized trial. JAMA 308:147–156
Sakuramoto S, Sasako M, Yamaguchi T et al (2007) Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 357:1810–1820
Uesaka K, Boku N, Fukutomi A et al (2016) Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet 388:248–257
Furuse J, Okusaka T, Boku N et al (2008) S-1 monotherapy as first-line treatment in patients with advanced biliary tract cancer: a multicenter phase II study. Cancer Chemother Pharmacol 62:849–855
Kobayashi S, Nagano H, Sakai D et al (2014) Phase I study of adjuvant gemcitabine or S-1 in patients with biliary tract cancers undergoing major hepatectomy: KHBO1003 study. Cancer Chemother Pharmacol 74:699–709
Morizane C, Okusaka T, Mizusawa J et al (2013) Randomized phase II study of gemcitabine plus S-1 versus S-1 in advanced biliary tract cancer: a Japan Clinical Oncology Group trial (JCOG 0805). Cancer Sci 104:1211–1216
Aoyama T, Yoshikawa T, Hayashi T et al (2013) Risk factors for 6-month continuation of S-1 adjuvant chemotherapy for gastric cancer. Gastric Cancer 16:133–139
Ebata T, Hirano S, Konishi M et al (2018) Randomized clinical trial of adjuvant gemcitabine chemotherapy versus observation in resected bile duct cancer. Br J Surg 105:192–202
Murakami Y, Uemura K, Sudo T et al (2009) Adjuvant gemcitabine plus S-1 chemotherapy improves survival after aggressive surgical resection for advanced biliary carcinoma. Ann Surg 250:950–956
Morizane C, Okusaka T, Mizusawa J et al (2018) Randomized phase III study of gemcitabine plus S-1 combination therapy versus gemcitabine plus cisplatin combination therapy in advanced biliary tract cancer: a Japan Clinical Oncology Group study (JCOG1113, FUGA-BT). J Clin Oncol 36:abstr. 205
Acknowledgements
We thank Shinichi Ohkawa, Shoji Nakamori and Yasuo Hamamoto for audit. This study was supported by Ministry of Health, Labour and Welfare of Japan (Grant Number: 22–50).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Masafumi Ikeda received honoraria from Taiho Pharmaceutical; Takuji Okusaka received research funding, consulting fee, and honoraria from Taiho Pharmaceutical; Hiroshi Ishii received research funding and honoraria from Taiho Pharmaceutical; Junji Furuse received research funding, consulting fee, and honoraria from Taiho Pharmaceutical; other authors have no conflict of interest.
About this article
Cite this article
Nakachi, K., Konishi, M., Ikeda, M. et al. Feasibility study of postoperative adjuvant chemotherapy with S-1 in patients with biliary tract cancer. Int J Clin Oncol 23, 894–899 (2018). https://doi.org/10.1007/s10147-018-1283-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-018-1283-6