Abstract
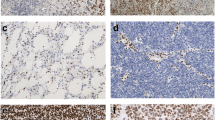
Vimentin is a marker of epithelial-mesenchymal transformation and indicates poor prognosis in various cancers, but its role in diffuse gliomas remains unknown. We investigated the vimentin expression of diffuse gliomas according to the upcoming 2021 WHO classification, its variations due to mutational status, and its prognostic effects. We analyzed vimentin immunohistochemistry in 315 gliomas: a test set (n = 164) and a validation set (n = 151). RNA-seq and mutational information from The Cancer Genome Atlas (TCGA, n = 422) were also used for validation. Vimentin was diffusely positive in astrocytic tumors but negative in oligodendroglial tumors (ODGs) and its expression was significantly higher in isocitrate dehydrogenase (IDH) wild-type tumors. High vimentin expression was correlated with poor prognosis (hazard ratio [HR]: 5.99), but it was dependent on the new WHO grade which reflects both histologic features and genetics (HR: 1.28). Using the significant difference in vimentin expression between ODGs and astrocytic tumors, the positive and negative predictive values of the vimentin-based diagnosis for ODGs were 93.5% and 97.8% in the validation set. Along with additional alpha-thalassemia/mental retardation, X-linked (ATRX) immunohistostaining, the values were 98.3% and 97.8%, respectively. Vimentin is a useful ancillary marker for identifying ODGs when combined with routine histochemistry markers.





Similar content being viewed by others
References
Ceccarelli M, Barthel FP, Malta TM et al (2016) Molecular profiling reveals biologically discrete subsets and pathways of progression in diffuse glioma. Cell 164:550–563. https://doi.org/10.1016/j.cell.2015.12.028
Louis DN, Perry A, Reifenberger G et al (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol (Berl) 131:803–820. https://doi.org/10.1007/s00401-016-1545-1
Louis DN, Wesseling P, Aldape K et al (2020) cIMPACT-NOW update 6: new entity and diagnostic principle recommendations of the cIMPACT-Utrecht meeting on future CNS tumor classification and grading. Brain Pathol Zurich Switz 30:844–856. https://doi.org/10.1111/bpa.12832
Phillips HS, Kharbanda S, Chen R et al (2006) Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell 9:157–173. https://doi.org/10.1016/j.ccr.2006.02.019
Verhaak RGW, Hoadley KA, Purdom E et al (2010) Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 17:98–110. https://doi.org/10.1016/j.ccr.2009.12.020
Behnan J, Finocchiaro G, Hanna G (2019) The landscape of the mesenchymal signature in brain tumours. Brain J Neurol 142:847–866. https://doi.org/10.1093/brain/awz044
Abaza MS, Shaban F, Narayan RK, Atassi MZ (1998) Human glioma associated intermediate filament proteins: over-expression, co-localization and cross-reactivity. Anticancer Res 18:1333–1340
Andersson D, Wilhelmsson U, Nilsson M et al (2013) Plasticity response in the contralesional hemisphere after subtle neurotrauma: gene expression profiling after partial deafferentation of the hippocampus. PLoS ONE 8:e70699. https://doi.org/10.1371/journal.pone.0070699
Hutchins JB, Casagrande VA (1989) Vimentin: changes in distribution during brain development. Glia 2:55–66. https://doi.org/10.1002/glia.440020107
Herpers MJ, Ramaekers FC, Aldeweireldt J et al (1986) Co-expression of glial fibrillary acidic protein- and vimentin-type intermediate filaments in human astrocytomas. Acta Neuropathol (Berl) 70:333–339. https://doi.org/10.1007/BF00686093
Kang YH, Han SR, Jeon H et al (2019) Nogo receptor–vimentin interaction: a novel mechanism for the invasive activity of glioblastoma multiforme. Exp Mol Med 51:1–15. https://doi.org/10.1038/s12276-019-0332-1
Nowicki MO, Hayes JL, Chiocca EA, Lawler SE (2019) Proteomic analysis implicates vimentin in glioblastoma cell migration. Cancers 11:466. https://doi.org/10.3390/cancers11040466
Nakopoulou L, Kerezoudi E, Thomaides T, Litsios B (1990) An immunocytochemical comparison of glial fibrillary acidic protein, S-100p and vimentin in human glial tumors. J Neurooncol 8:33–40. https://doi.org/10.1007/BF00182084
Dehghani F, Schachenmayr W, Laun A, Korf HW (1998) Prognostic implication of histopathological, immunohistochemical and clinical features of oligodendrogliomas: a study of 89 cases. Acta Neuropathol (Berl) 95:493–504. https://doi.org/10.1007/s004010050830
Lin L, Wang G, Ming J et al (2016) Analysis of expression and prognostic significance of vimentin and the response to temozolomide in glioma patients. Tumour Biol J Int Soc Oncodevelopmental Biol Med 37:15333–15339. https://doi.org/10.1007/s13277-016-5462-7
Lassman AB, Roberts-Rapp L, Sokolova I et al (2019) Comparison of biomarker assays for EGFR: implications for precision medicine in patients with glioblastoma. Clin Cancer Res 25:3259–3265. https://doi.org/10.1158/1078-0432.CCR-18-3034
Pirker R, Pereira JR, von Pawel J et al (2012) EGFR expression as a predictor of survival for first-line chemotherapy plus cetuximab in patients with advanced non-small-cell lung cancer: analysis of data from the phase 3 FLEX study. Lancet Oncol 13:33–42. https://doi.org/10.1016/S1470-2045(11)70318-7
Santosh N, McNamara KK, Beck FM, Kalmar JR (2019) Expression of cornulin in oral premalignant lesions. Oral Surg Oral Med Oral Pathol Oral Radiol 127:526–534. https://doi.org/10.1016/j.oooo.2019.02.003
Cerami E, Gao J, Dogrusoz U et al (2012) The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2:401–404. https://doi.org/10.1158/2159-8290.CD-12-0095
Gao J, Aksoy BA, Dogrusoz U et al (2013) Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 6:pl1. https://doi.org/10.1126/scisignal.2004088
Weller M, van den Bent M, Tonn JC et al (2017) European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol 18:e315–e329. https://doi.org/10.1016/S1470-2045(17)30194-8
Koperek O, Gelpi E, Birner P et al (2004) Value and limits of immunohistochemistry in differential diagnosis of clear cell primary brain tumors. Acta Neuropathol (Berl) 108:24–30. https://doi.org/10.1007/s00401-004-0856-9
Herpers MJ, Budka H (1984) Glial fibrillary acidic protein (GFAP) in oligodendroglial tumors: gliofibrillary oligodendroglioma and transitional oligoastrocytoma as subtypes of oligodendroglioma. Acta Neuropathol (Berl) 64:265–272. https://doi.org/10.1007/BF00690392
Reuss DE, Sahm F, Schrimpf D et al (2015) ATRX and IDH1-R132H immunohistochemistry with subsequent copy number analysis and IDH sequencing as a basis for an “integrated” diagnostic approach for adult astrocytoma, oligodendroglioma and glioblastoma. Acta Neuropathol (Berl) 129:133–146. https://doi.org/10.1007/s00401-014-1370-3
Gillet E, Alentorn A, Doukouré B et al (2014) TP53 and p53 statuses and their clinical impact in diffuse low grade gliomas. J Neurooncol 118:131–139. https://doi.org/10.1007/s11060-014-1407-4
Gomes FC, Paulin D, Moura Neto V (1999) Glial fibrillary acidic protein (GFAP): modulation by growth factors and its implication in astrocyte differentiation. Braz J Med Biol Res Rev Bras Pesqui Medicas Biol 32:619–631. https://doi.org/10.1590/s0100-879x1999000500016
Louis DN (2009) Non-neoplastic diseases of the central nervous system. American Registry of Pathology, Washington, DC
Satelli A, Li S (2011) Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell Mol Life Sci CMLS 68:3033–3046. https://doi.org/10.1007/s00018-011-0735-1
Zhao J, Zhang L, Dong X et al (2018) High expression of vimentin is associated with progression and a poor outcome in glioblastoma. Appl Immunohistochem Mol Morphol 26:337–344. https://doi.org/10.1097/PAI.0000000000000420
Wang Q, Hu B, Hu X et al (2017) Tumor evolution of glioma-intrinsic gene expression subtypes associates with immunological changes in the microenvironment. Cancer Cell 32:42-56.e6. https://doi.org/10.1016/j.ccell.2017.06.003
Bhat KPL, Balasubramaniyan V, Vaillant B et al (2013) Mesenchymal differentiation mediated by NF-κB promotes radiation resistance in glioblastoma. Cancer Cell 24:331–346. https://doi.org/10.1016/j.ccr.2013.08.001
Fedele V, Dai F, Masilamani AP et al (2017) Epigenetic regulation of ZBTB18 promotes glioblastoma progression. Mol Cancer Res MCR 15:998–1011. https://doi.org/10.1158/1541-7786.MCR-16-0494
Wu G, Diaz AK, Paugh BS et al (2014) The genomic landscape of diffuse intrinsic pontine glioma and pediatric non-brainstem high-grade glioma. Nat Genet 46:444–450. https://doi.org/10.1038/ng.2938
Ellison DW, Hawkins C, Jones DTW et al (2019) cIMPACT-NOW update 4: diffuse gliomas characterized by MYB, MYBL1, or FGFR1 alterations or BRAFV600E mutation. Acta Neuropathol (Berl) 137:683–687. https://doi.org/10.1007/s00401-019-01987-0
Ebrahimi A, Skardelly M, Bonzheim I et al (2016) ATRX immunostaining predicts IDH and H3F3A status in gliomas. Acta Neuropathol Commun 4:60. https://doi.org/10.1186/s40478-016-0331-6
Acknowledgements
This study was supported by a grant from Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health and Welfare, Republic of Korea [grant no. HI14C1277] and the National Cancer Center, Republic of Korea (NCC-1810861-1).
Author information
Authors and Affiliations
Contributions
J-KW and S-HP designed the study. J-KW, S-IK, and S-HP reviewed histologic slides, drew annotations and acquired data for qualitative analysis. KL acquired data for quantitative analysis using positive pixel count v9 algorithm. C-KP and SHC carried out acquisition and analysis of clinical data. SL and HY acquired data from targeted brain tumor-related gene panel sequencing. S-IK and KL performed statistical analysis. S-IK, KL, JB, SL, HY, S-HP, and J-KW wrote the manuscript. All authors reviewed and edited the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kim, SI., Lee, K., Bae, J. et al. Revisiting vimentin: a negative surrogate marker of molecularly defined oligodendroglioma in adult type diffuse glioma. Brain Tumor Pathol 38, 271–282 (2021). https://doi.org/10.1007/s10014-021-00411-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10014-021-00411-4