Abstract
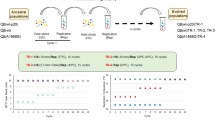
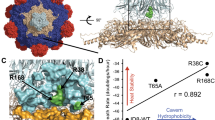
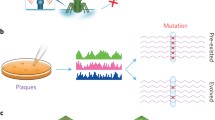
A population’s growth rate is determined by multiple ‘life history traits’. To quantitatively determine which life history traits should be improved to allow a living organism to adapt to an inhibitory environment is an important issue. Previously, we conducted thermal adaptation experiments on the RNA bacteriophage Qβ using three independent replicates and reported that all three end-point populations could grow at a temperature (43.6°C) that inhibited the growth of the ancestral strain. Even though the fitness values of the endpoint populations were almost the same, their genome sequence was not, indicating that the three thermally adapted populations may have different life history traits. In this study, we introduced each mutation observed in these three end-point populations into the cDNA of the Qβ genome and prepared three different mutants. Quantitative analysis showed that they tended to increase their fitness by increasing the adsorption rate to their host, shortening their latent period (i.e., the duration between phage infection and progeny release), and increasing the burst size (i.e., the number of progeny phages per infected cell), but all three mutants decreased their thermal stability. However, the degree to which these traits changed differed. The mutant with the least mutations showed a smaller decrease in thermal stability, the largest adsorption rate to the host, and the shortest latent period. These results indicated that several different adaptive routes exist by which Qβ can adapt to higher temperatures, even though Qβ is a simple RNA bacteriophage with a small genome size, encoding only four genes.





Similar content being viewed by others
References
Stearns SC (1976) Life-history tactics: a review of the ideas. Q Rev Biol 51:3–47
Futuyma DJ (2005) How to be fit: reproductive success. Evolution. Sinauer associates Inc, Sunderland, pp 405–428
Gadgil M, Bossert WH (1970) Life historical consequences of natural selection. Am Nat 104:1–24
Stearns SC (1992) Evolutionary explanation. The evolution of life histories. Oxford University Press, New York, pp 9–19
Reznick D (1985) Costs of reproduction: an evaluation of the empirical evidence. Oikos 44:257
Stearns SC (1989) Trade-offs in life-history evolution. Func Ecol 3:259–268
De Paepe M, Taddei F (2006) Viruses’ life history: towards a mechanistic basis of a trade-off between survival and reproduction among phages. PLoS Biol 4:e193
Garcia-Villada L, Drake JW (2013) Experimental selection reveals a trade-off between fecundity and lifespan in the coliphage Qβ. Open Biol 3:130043
Kashiwagi A, Sugawara R, Sano Tsushima F, Kumagai T, Yomo T (2014) Contribution of silent mutations to thermal adaptation of RNA bacteriophage Qβ. J Virol 88:11459–11468
Van Duin J, Tsareva N (2006) Single-stranded RNA phages. In: Calendar R (ed) The bacteriophages. Oxford University Press, New York, pp 175–196
Kashiwagi A, Yomo T (2011) Ongoing phenotypic and genomic changes in experimental coevolution of RNA bacteriophage Qβ and Escherichia coli. PLoS Genet 7:e1002188
Kashiwagi A, Sakurai T, Tsuru S, Ying BW, Mori K, Yomo T (2009) Construction of Escherichia coli gene expression level perturbation collection. Metab Eng 11:56–63
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A 74:5463–5467
Kropinski AM, Mazzocco A, Waddell TE, Lingohr E, Johnson RP (2009) Enumeration of bacteriophages by double agar overlay plaque assay. In: Clokie MR, Kropinski AM (eds) Bacteriophages methods and protocols, Volume 1: isolation, characterization, and interactions. Methods in molecular biology. Humana Press, New York, pp 69–76
Taylor JR (1997) Propagation of uncertainties. In: Introduction to error analysis: the study of uncertainties in physical measurements. University Science Books, Philadelphia
Inomata T, Kimura H, Hayasaka H, Shiozaki A, Fujita Y, Kashiwagi A (2012) Quantitative comparison of the RNA bacteriophage Qβ infection cycle in rich and minimal media. Arch Virol 157:2163–2169
Zar JH (2010) Biostatistical analysis, 5th edn. Pearson education Inc, Upper Saddle River
Eigen M, Schuster P (1977) The hypercycle. A principle of natural self-organization. Part A: emergence of the hypercycle. Naturwissenschaften 64:541–565
Gorzelnik KV, Cui Z, Reed CA, Jakana J, Young R, Zhang J (2016) Asymmetric cryo-EM structure of the canonical Allolevivirus Qβ reveals a single maturation protein and the genomic ssRNA in situ. Proc Natl Acad Sci USA 113:11519–11524
Ellis EL, Delbruck M (1939) The growth of bacteriophage. J Gen Physiol 22:365–383
Karnik S, Billeter M (1983) The lysis function of RNA bacteriophage Qβ is mediated by the maturation (A2) protein. EMBO J 2:1521–1526
Dai X, Li Z, Lai M, Shu S, Du Y, Zhou ZH, Sun R (2017) In situ structures of the genome and genome-delivery apparatus in a single-stranded RNA virus. Nature 541:112–116
Rumnieks J, Tars K (2017) Crystal structure of the maturation protein from bacteriophage Qβ. J Mol Biol 429:688–696
Blumenthal T, Landers TA, Weber K (1972) Bacteriophage Q replicase contains the protein biosynthesis elongation factors EF Tu and EF Ts. Proc Natl Acad Sci U S A 69:1313–1317
Arribas M, Kubota K, Cabanillas L, Lazaro E (2014) Adaptation to fluctuating temperatures in an RNA virus is driven by the most stringent selective pressure. PLoS ONE 9:e100940
Goldhill DH, Turner PE (2014) The evolution of life history trade-offs in viruses. Curr Opin Virol 8:79–84
Abedon ST, Hyman P, Thomas C (2003) Experimental examination of bacteriophage latent-period evolution as a response to bacterial availability. Appl Environ Microbiol 69:7499–7506
Heineman RH, Brown SP (2012) Experimental evolution of a bacteriophage virus reveals the trajectory of adaptation across a fecundity/longevity trade-off. PLoS ONE 7:e46322
Acknowledgments
We would like to thank Shoko Kida, Ryu Sugawara, Saki Yamada, Asami Yamazaki, and Kazuya Kishi for the technical assistance. We are grateful to Dr. Takahiro Toba for his cooperation.
Funding
This work was supported in part by JSPS KAKENHI (Grant Number 26440194) and the Hirosaki University Institutional Research Grant for Young Scientists.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Handling Editor: Chan-Shing Lin.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kashiwagi, A., Kadoya, T., Kumasaka, N. et al. Influence of adaptive mutations, from thermal adaptation experiments, on the infection cycle of RNA bacteriophage Qβ. Arch Virol 163, 2655–2662 (2018). https://doi.org/10.1007/s00705-018-3895-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-018-3895-6