Abstract
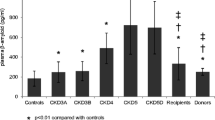
The pathological changes of Alzheimer’s disease include the deposition of amyloid β protein (Aβ) as senile plaques in the brain. We hypothesized that the rapid removal of Aβs from the blood may act as a peripheral Aβ drainage sink from the brain. In this study, the plasma Aβ concentrations and the cognitive functions were investigated for in 57 patients on hemodailysis (69.4 ± 3.8 years), 26 renal-failure patients without hemodialysis (66.6 ± 14.7 years), and 17 age-matched healthy controls (66.6 ± 4.1 years). The concentrations of plasma Aβs increased along with the decline of renal functions. Moreover, the renal-failure patients without hemodialysis and with poorer renal functions showed lower cognitive functions. The plasma concentrations of Aβ1-42 correlated with serum creatinine (P < 0.001) and Mini-Mental-State Examination scores (P = 0.017). The dialyzers effectively removed Aβs in the blood during hemodialysis sessions. The plasma Aβ concentrations showed steady or slightly decreasing along with duration of hemodialysis. The total amount of Aβs removed during a hemodialysis session was calculated to be comparable to the Aβs dissolved in the blood and the cerebrospinal fluid. The MMSE scores of the hemodialysis patients showed no clear decrease in longer hemodialysis duration. Therefore, the therapeutic approach for Alzheimer’s disease by removing Aβs from the blood is worthy of further investigation, including whether or not Aβs in the brain decrease.












Similar content being viewed by others
References
Andreasen N, Minthon L, Davidsson P, Vanmechelen E, Vanderstichele H, Winblad B, Blennow K (2001) Evaluation of CSF-tau and CSF-Aβ42 as diagnostic markers for Alzheimer disease in clinical practice. Arch Neurol 58:373–379
Bell RD, Sagare AP, Friedman AE, Bedi GS, Holtzman DM, Deane R, Zlokovic BV (2007) Transport pathways for clearance of human Alzheimer’s amyloid beta-peptide and apolipoproteins E and J in the mouse central nervous system. J Cereb Blood Flow Metab 27(5):909–918
Boada M, Ortiz P, Anaya F, Hernández I, Muñoz J, Núñez L, Olazarán J, Roca I, Cuberas G, Tárraga L, Buendia M, Pla RP, Ferrer I, Páez A (2009) Amyloid-targeted therapeutics in Alzheimer’s disease: use of human albumin in plasma exchange as a novel approach for Abeta mobilization. Drug News Perspect 22:325–329
Donahue JE, Flaherty SL, Johanson CE, Duncan JA 3rd, Silverberg GD, Miller MC, Tavares R, Yang W, Wu Q, Sabo E, Hovanesian V, Stopa EG (2006) RAGE, LRP-1, and amyloid-beta protein in Alzheimer’s disease. Acta Neuropathol 4:405–415
Folstein MF, Folstein SE, McHugh PR (1975) Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Ghiso J, Calero M, Matsubara E, Governale S, Chuba J, Beavis R, Wisniewski T, Frangione B (1997) Alzheimer’s soluble amyloid β is a normal component of human urine. FEBS Lett 408:105–108
Ghiso J, Shayo M, Calero M, Ng D, Tomidokoro Y, Gandy S, Rostagno A, Frangione B (2004) Systemic catabolism of Alzheimer’s Aβ40 and Aβ42. J Biol Chem 279:45897–45908
Hock C, Konietzko U, Streffer JR, Tracy J, Signorell A, Müller-Tillmanns B, Lemke U, Henke K, Moritz E, Garcia E, Wollmer MA, Umbricht D, de Quervain DJ, Hofmann M, Maddalena A, Papassotiropoulos A, Nitsch RM (2003) Antibodies against beta-amyloid slow cognitive decline in Alzheimer’s disease. Neuron 38:547–554
Hung LW, Ciccotosto GD, Giannakis E, Tew DJ, Perez K, Masters CL, Cappai R, Wade JD, Barnham KJ (2008) Amyloid-b peptide (Ab) neurotoxicity is modulated by the rate of peptide aggregation: Ab dimers and trimers correlate with neurotoxicity. J Neurosci 28(46):11950–11958
Jin R, Grunkemeier GL, Brown JR, Furnary AP (2008) Estimated glomerular filtration rate and renal function. Ann Thorac Surg 86:4–11
Kawaguchi K, Kitaguchi N, Nakai S, Murakami K, Asakura K, Mutoh T, Fujita Y, Sugiyama S (2010) Novel therapeutic approach for Alzheimer’s disease by removing amyloid beta protein from the brain with an extracorporeal removal system. J Artif Organs 13:31–37
Kitaguchi N, Kawaguchi K, Nakai S, Murakami K, Ito S, Hoshino H, Hori H, Ohashi A, Shimano Y, Suzuki N, Yuzawa Y, Mutoh T, Sugiyama S (2011) Reduction of Alzheimer’s disease amyloid-β in plasma by hemodialysis and its relation to cognitive functions. Blood Purif 32:57–62
Koumi H, Maeda A, Yamamoto A, Kato Y, Okamura K, Sonoda K, Ando E, Kishikawa Y (2010) The sensitivity and specificity of Japanese version of the Mini-Mental Sate Examination. Bull Fac Soc Welfare Hanazono Univ 18:91–95
Kuo YM, Emmerling MR, Vigo-Pelfrey C, Kasunic TC, Kirkpatrick JB, Murdoch GH, Ball MJ, Roher AE (1996) Water-soluble Abeta (N-40, N-42) oligomers in normal and Alzheimer disease brains. J Biol Chem 271:4077–4081
Lopez OL, Kuller LH, Mehta PD, Becker JT, Gach HM, Sweet RA, Chang YF, Tracy R, DeKosky ST (2008) Plasma amyloid levels and the risk of AD in normal subjects in the cardiovascular health study. Neurology 70:1664–1671
Matsuoka Y, Saito M, LaFrancois J, Saito M, Gaynor K, Olm V, Wang L, Casey E, Lu Y, Shiratori C, Lemere C, Duff K (2003) Novel therapeutic approach for the treatment of Alzheimer’s disease by peripheral administration of agents with an affinity to β-amyloid. J Neurosci 23:29–33
Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, Yarasheski KE, Bateman RJ (2010) Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science 330(6012):1774
Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Liao Z, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C, Walker S, Wogulis M, Yednock T, Games D, Seubert P (1999) Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature 400:173–177
Selkoe DJ (2001) Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev 81:741–766
Silverberg GD, Miller MC, Messier AA, Majmudar S, Machan JT, Donahue JE, Stopa EG, Johanson CE (2010) Amyloid deposition and influx transporter expression at the blood-brain barrier increase in normal aging. J Neuropathol Exp Neurol 69:98–108
Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ (2002) Naturally secreted oligomers of amyloid b protein potently inhibit hippocampal long-term potentiation in vivo. Nature 416:535–539
Yaffe K, Ackerson L, Kurella Tamura M, Le Blanc P, Kusek JW, Sehgal AR, Cohen D, Anderson C, Appel L, Desalvo K, Ojo A, Seliger S, Robinson N, Makos G (2010) Chronic kidney disease and cognitive function in older adults: findings from the chronic renal insufficiency cohort cognitive study. J Am Geriatr Soc 58:338–345
Acknowledgments
The authors sincerely thank Dr. Shigenobu Nakamura for his fruitful discussions. The authors also thank Ms. Yukari Murata and Sachi Oguri for their technical assistance. This work was partly supported by KAKENHI (20509008 and 23500531) and the Smoking Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
M. Kato and K. Kawaguchi contributed equally.
Rights and permissions
About this article
Cite this article
Kato, M., Kawaguchi, K., Nakai, S. et al. Potential therapeutic system for Alzheimer’s disease: removal of blood Aβs by hemodialzyers and its effect on the cognitive functions of renal-failure patients. J Neural Transm 119, 1533–1544 (2012). https://doi.org/10.1007/s00702-012-0844-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-012-0844-5