Abstract
Objective
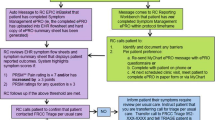
Clinic-based collection of patient-reported outcome (PRO) quantifying symptom burden provide crucial information for effective care. We have pioneered point-of-care electronic assessment using the Edmonton Symptom Assessment Scale (ESAS) with direct linkage to the electronic medical record (EMR) which has been readily adopted by our oncology patients. As some patients may complete more than one ESAS per day in different clinics, the goal of the current analyses was to compare the within-patient congruence of ESAS assessments completed on the same day.
Methods
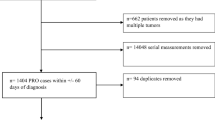
A total of 9621 ESAS records from 4021 patients of the Supportive Care Medicine and Radiation Oncology clinics between February and November 2017 were retrieved from the EMR. Patients completed the ESAS-r-CSS, which added sleep disturbance, constipation, and spiritual well-being domains to the standard ESAS-r.
Results
A total of 65 patients provided more than one ESAS report within the same day. The data were curated, removing those sporadic missing data and those with obvious technical error. This process left 130 samples for analysis. There was no statistical difference among different ESAS collection intervals for domains of tiredness, nausea, appetite, overall well-being, spiritual well-being, constipation, and difficulty sleeping, but there was a significant difference for pain, drowsiness, shortness of breath, depression, and anxiety. Repeat tests that occurred within 1 h of one another demonstrated higher congruence than those completed over longer periods.
Conclusion
Patients reported significant worsening of several symptoms over the course of the day, with greatest concordance observed within smaller time periods.
Similar content being viewed by others
References
Basch E, Deal AM, Kris MG et al (2016) Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol 34:557–565
Cleeland CS, Wang XS, Shi Q, Mendoza TR, Wright SL, Berry MD, Malveaux D, Shah PK, Gning I, Hofstetter WL, Putnam JB Jr, Vaporciyan AA (2011) Automated symptom alerts reduce postoperative symptom severity after cancer surgery: a randomized controlled clinical trial. J Clin Oncol 29:994–1000
Basch E, Deal AM, Kris MG, Scher HI, Hudis CA, Sabbatini P, Rogak L, Bennett AV, Dueck AC, Atkinson TM, Chou JF, Dulko D, Sit L, Barz A, Novotny P, Fruscione M, Sloan JA, Schrag D (2016 Feb 20) Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol 34(6):557–565
Basch E, Deal AM, Dueck AC, Scher HI, Kris MG, Hudis C, Schrag D (2017 Jul 11) Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA 318(2):197–198
Johnstone PAS, Lee J, Zhou JM, Ma Z, Portman D, Jim H, Yu HM (2017 Sep) A modified Edmonton Symptom Assessment Scale for symptom clusters in radiation oncology patients. Cancer Med 6(9):2034–2041
Portman D, Thirlwell S, Atkins L et al (2017) An e-App to foster symptom documentation and intervention. J Pall Med 20(4):A-22 (#46)
Watanabe SM, Nekolaichuk C, Baumont C et al (2011) A multicenter study comparing two numerical versions of the Edmonton Symptom Assessment System in palliative care patients. J Pain Symptom Manag 41(2):456–468
Chang VT, Hwang SS, Feuerman M (2000) Validation of the Edmonton symptom assessment scale. Cancer 88(9):2164–2171
Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K (1991) The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care 7:6–9
Dudgeon DJ, Harlos M, Clinch JJ (1999) The Edmonton Symptom Assessment Scale (esas) as an audit tool. J Palliat Care 15:14–19
Nelson JE, Meier DE, Oei EJ, Nierman DM, Senzel RS, Manfredi PL, Davis SM, Morrison RS (2001) Self-reported symptom experience of critically ill cancer patients receiving intensive care. Crit Care Med 29:277–282
Rees E, Hardy J, Ling J, Broadley K, A’Hern R (1998) The use of the Edmonton Symptom Assessment Scale (ESAS) within a palliative care unit in the UK. Palliat Med 12(2):75–82
Richardson LA, Jones GW (2009 Jan) A review of the reliability and validity of the Edmonton Symptom Assessment System. Curr Oncol 16(1):55–64
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Johnstone has nothing to disclose. Dr. Bulls has nothing to disclose. Dr. Lee has nothing to disclose. Dr. Zhou has nothing to disclose. Dr. Portman has nothing to disclose. Dr. Yu reports personal fees from UpToDate, Inc. Dr. Jim has nothing to disclose.
Rights and permissions
About this article
Cite this article
Johnstone, P.A.S., Bulls, H.W., Zhou, JM. et al. Congruence of multiple patient-related outcomes within a single day. Support Care Cancer 27, 867–872 (2019). https://doi.org/10.1007/s00520-018-4372-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-018-4372-1