Summary
Background
The aim of this systematic review was to update scientific knowledge concerning the safety of neuromuscular electrical stimulation (NMES) to increase exercise capacity and prevent cardiac cachexia in patients with implantable cardioverter defibrillators (ICDs).
Methods
A systematic review including the electronic databases PubMed, MEDLINE, and SCOPUS was conducted for the time period from 1966 to March 31, 2016.
Results
Only four articles fulfilled the inclusion criteria (three original articles/safety studies and one case report). The three (safety) studies used NMES to increase muscle strength and/or endurance capacity of the thighs. NMES did not show electromagnetic interference (EMI) with ICD function. EMI was described in a case report of 2 patients with subpectoral ICDs and application of NMES on abdominal muscles.
Conclusion
This review indicates that NMES may be applied in cardiac ICD patients if 1) individual risks (e. g., pacing dependency, acute heart failure, unstable angina, ventricular arrhythmic episode in the last 3 months) are excluded by performing a safety check before starting NMES treatment and 2) “passive” exercise using NMES is performed only for thighs and gluteal muscles in 3) compliant ICD patients (especially for home-based NMES) and 4) the treatment is regularly supervised by a physician and the device is examined after the first use of NMES to exclude EMI. Nevertheless, further studies including larger sample sizes are necessary to exclude any risk when NMES is used in this patient group.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Typical symptoms of severe congestive chronic heart failure (CHF) are dyspnea, edema, weakness, and reduced functional capacity of skeletal muscles, which can result in cardiac cachexia [1–4]. Cardiac cachexia is a serious complication of CHF with a high morbidity and mortality, and is characterized by significant weight loss and muscle wasting [1, 2]. CHF-related muscle wasting is the result of an ongoing imbalance in the activation of anabolic and catabolic pathways [3, 5, 6]. This imbalance is caused by a series of immunologic, metabolic, and neuro-hormonal processes [2, 6, 7]. Efficient multidisciplinary care seems to be pivotal for disease management in this patient group [8].
Muscular strength of the thighs has been shown to be a predictor of long-term survival in CHF [1, 9, 10]. Patients with advanced CHF (New York Heart Association, NYHA, class IV) are generally excluded from active exercise to maintain muscle mass and functional capacity of skeletal muscle, namely endurance capacity and muscular strength [1, 10]. The physical treatment modality of neuromuscular electrical stimulation (NMES)—evoking muscle contractions by using electrical stimulation—is an established method to prevent atrophy of skeletal muscles [11–13]. It can be seen as an alternative option to active endurance and strength exercise for patients who are not allowed (contraindication) or unable (immobilization) to perform active exercise [14–17]. NMES leads to improvements in endurance capacity, muscular strength, and cross-sectional area of thigh muscles in patients with CHF, and has therefore been shown to be an effective option for “passive” exercise in these patients [17–19]. NMES has been described to effectively substitute, promote, or complement active exercise and increase adherence to rehabilitation protocols, especially for end-stage cardiac patients [17, 20]. Nevertheless, despite its benefits for exercise capacity and quality of life, NMES is currently underutilized in patients with CHF [21].
However, about 20 percent of CHF patients carry active electronic implants such as implantable cardioverter defibrillators (ICDs). Therefore, there is an existing and remaining concern of whether NMES can be applied in these patients, due to fear of electromagnetic interference (EMI). EMI can cause oversensing and lead to inappropriate therapies in ICD patients, resulting in arrhythmias and/or painful shocks [22–24]. These patients are generally advised to avoid exposure to electrical currents and electromagnetic sources, even though ICDs seem to be less sensitive to interference than pacemakers [25].
Pilot safety studies performed at the beginning of this century, designed to test the safety of NMES in patients with bipolar sensing by ICD lead systems, have shown that NMES treatment of knee extensor and flexor muscles seems to be safe and feasible in patients with bipolar sensing ICDs, providing that an individual risk is excluded before application [26, 27]. Nevertheless, several reports exist of inappropriate therapies/shocks due to interactions with electronic devices or electrical stimulation therapy leading to EMI [28–30].
This review aims to summarize and update knowledge from the scientific literature concerning NMES of thighs in ICD patients.
Methods
A systematic review of the existing scientific literature was performed, including the electronic databases PubMed, MEDLINE, and SCOPUS. Electronic searches were conducted for the time period from 1966 to March 31, 2016. Trials with the keywords “Implanted Cardioverter Defibrillator”, “ICD”, “Neuromuscular stimulation”, “NMES”, “Functional electrical stimulation”, “FES”, and “Functional muscular stimulation” were extracted and considered for inclusion in the review. Furthermore, the combinations “Physical therapy and ICD and EMI” and “Physical therapy and ICD and Interference”, were also checked for inclusion in the review.
Studies were eligible for inclusion if they met the following criteria: original articles/safety studies, pilot studies, case reports, and reviews concerning the topic NMES in ICD patients were included. The restriction placed on language was that only English and German articles were included. The systematic literature search was performed separately by two independent researchers. Integration of their individual findings was supervised by two senior researchers [31, 32]. Author searches for key experts in the field were conducted for additional relevant articles. Furthermore, reference lists of each relevant publication were searched for additional information.
Results
Using the described search strategy, a total of 725 publications were found and screened for eligibility by title and abstract. 721 were rejected as non-includable or duplicates, and four studies were selected for full-text analysis (Fig. 1).
Of these, four fulfilled the inclusion criteria of being:
-
Original articles (full text available in English or German),
-
Safety studies, pilot studies, case reports, or reviews.
Studies were excluded because of the following criteria:
-
Full text not available in English or German,
-
Reporting an intervention using transcutaneous electrical nerve stimulation (TENS, for treatment of pain) or other currents for analgesia, but no NMES.
Case report
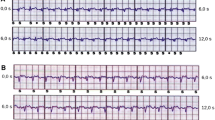
Wayar et al. reported on two male patients with pectoral implanted ICDs (Ventak Mini III® and Ventak AV III DR®) and neuromuscular stimulation of abdominal muscles [33]. In both patients, NMES using commercial units (5.0–11.0 V, 7.3–10 mA, 55–75 Hz/biphasic) provoked EMI, resulting in ICD discharge due to misinterpreting electrical signals as cardiac signals in the ventricular fibrillation (VF) zone (Table 1; [33]).
Safety studies
Crevenna et al. performed the first safety study [27]. Eight patients had subpectorally implanted ICDs with transvenous bipolar sensing leads and received different types (different current forms) of electrical stimulation of trapezoid and thigh muscles, namely impulse galvanization (IG50); frequency modulation (FM); high-frequency transcutaneous electrical nerve stimulation (HF-TENS) and low-frequency transcutaneous electrical nerve stimulation (LF-TENS) of trapezoid muscles; “E200” (impulses of a rise duration of 200 ms and pulse duration of 270 ms at a frequency of 0.44 Hz); “aS” (pulse duration of 400 µs with a threshold duration of approximately 6.5 s at a frequency of 66.7 Hz); “aS1” (pulse duration of 400 µs and a threshold duration of 3.6 Hz at a frequency of 66.7 Hz); and a (specially programmed) electrical current (“FIB”) comprising triangular impulses over 60 ms with an interval of 200 ms, mimicking an episode of tachycardia in the ICD detection zone. Furthermore, two different commercially available devices for home therapy for increasing endurance capacity (Stiwell 1200 (Med-El, Innsbruck, Austria) and Compex 2 [Compex SA, Ecublens, Switzerland]) were tested.
The results showed that electrical stimulation was well tolerated in all participants and no clinical side effects were reported. No interference with ventricular sensing during electrical stimulation was seen in 5 of 8 patients. No EMI was caused upon stimulating the thigh musculature using home therapy devices or during stimulation protocols “E200”, “aS”, or “aS1”. Mimicking a tachycardia by using “FIB” to thigh muscles was enforced in 2 patients. EMI of ventricular sensing was also caused by stimulating trapezoid muscles during FM in 1 patient and during LF-TENS in 2 patients. The authors advise investigating EMI before starting NMES in patients with ICDs (Table 1; [27]).
In another study, Crevenna et al. assessed the safety of long-term NMES in patients with ICDs [26]. Six patients with subpectoral ICDs were exposed to long-term NMES of thigh muscles. Four inpatients received NMES to increase muscle strength (biphasic symmetric pulses with pulse duration of ±400 ms at a frequency of 63.3 Hz; 3.5 s on, 4.5 s off). Two outpatients performed NMES as home treatment to increase endurance capacity (biphasic symmetric pulses of 500-ms pulse width at a frequency of 15 Hz; 2 s on and 4 s off). During NMES, all participants together received 14,139,799 biphasic electrical pulses and 412,425 on-phases without adverse events. No abnormalities of ICD function were recorded after the stimulation period in any patients. In a safety procedure, NMES was applied under supervised conditions to evaluate the individual risk. Symptom-limited NMES was performed and the ICD was interrogated online for potential occurrences of EMI. The results of this indicated that long-term NMES of thigh muscles seems to be safe in patients with ICDs, providing that an individual risk was excluded prior to start of NMES therapy (Table 1; [26]).
Kamiya et al. recently performed a safety study with NMES in 27 patients with left pectoral ICDs [34]. Medium-frequent NMES of knee flexors and extensors with alternating sinusoidal current (2.5 kHz) for 20 minutes in bursts with a carrier frequency of 50 Hz and impulse trains for 5 s and pauses for 5 s was used at individual highest tolerable intensities. In this case, NMES of thigh muscles was applied to the participants without any occurrence of EMI. Additionally, EMI between ICDs and NMES application was not observed. The authors therefore concluded that NMES of thigh and calf musculature can be safely applied to patients with an ICD (Table 1; [34]).
Discussion
Muscular strength of thighs has been shown to be a predictor of long-term survival in CHF [1, 9, 10]. As Parissis et al. have summarized, functional electrical stimulation of thighs and calf muscles is the only alternative mode of exercise in patients with CHF [18].
Literature about NMES in patients with ICDs is very rare. The reason for this seems to be that ICDs are generally seen as a contraindication for the use of NMES by the community. Nevertheless, NMES has been described to be an effective and safe treatment option for ICD patients provided certain safety precautions are considered [26, 27, 34]. In the scientific literature, there are only four articles describing the safety of NMES in patients with pectoral ICDs. Three of them showed the safety of NMES when applied to lower extremities in order to improve muscle strength and endurance capacity [26, 27, 34]. Crevenna et al. reported feasibility and safety of NMES in ICD patients if individual risks were checked prior to treatment initiation [26, 27].
In contrast, inappropriate electrode positioning for NMES can provoke disturbances of ICDs [33]. In one study, 2 patients autonomously applied the stimulation too close to the implantation site of the pectoral ICD, namely to the abdominal musculature, causing discharge of the ICD by signals in the VF zone [33]. Nevertheless, at that time, ICD programming had usually been very strict, e. g., short detection of VF and shock delivery at the first stage in the VF zone. Nowadays, prolonged detection programming and shock delivery only if antitachycardia pacing etc. fail may prevent many inappropriate shocks. Therefore, it might be doubtful that modern ICDs can be disturbed by NMES in the abdominal region. Nevertheless, further studies are needed to answer this question.
Unfortunately, the terms TENS (for pain treatment) and NMES are not clearly distinguished by all publishing authors. TENS is a non-pharmacologic treatment for pain relief, applied in the form of low-frequency (<12 Hz) or high-frequency TENS (50–100 Hz). Some case reports present discharge of pectoral or abdominal ICDs in patients during use of TENS for pain treatment in the lumbar, trapezoidal, and abdominal musculature, or chest or midback area [27–30, 35]. In another case report, TENS of the sacral region provoked an ICD discharge, although the electrodes were 12 inches away from the ICD pulse generator [36]. In an exploratory study, 30 patients with implanted defibrillators (ICDs) underwent TENS treatment above the mammillae and the anterior superior iliac spine after programming the ICD to monitoring mode. Due to possible consequences of inexact sensing, the authors do not advise the application of TENS in patients with an ICD [37]. Pain management using electrotherapy, e. g., TENS, is substituted by pharmacological treatment and should be avoided in patients with ICDs.
Options to improve muscle strength and endurance capacity in patients with end-stage heart failure are rather limited. In most cases, patients with CHF are discouraged from performing active exercise to maintain muscle mass and functional capacity of skeletal muscle, namely endurance capacity and muscular strength [1, 11]. In Austria, NMES treatment of patients suffering from chronic diseases has a long tradition [17, 20, 26, 27, 38–42]. Depending on the exercise goal, different commercially available therapy devices are used with direct or alternating current (biphasic) between 8–63.3 Hz, and, according to this, adjustable pulse durations and session length (usually 20–30 min).
This systematic review of existing scientific literature indicates that NMES treatment of thigh muscles seems to be safe and feasible in CHF patients with bipolar ICDs. Nevertheless, risk analyses to detect harmful events require a large sample size in order to arrive at valid predictions for the community, and such high powered and large safety studies are yet to be realized. We therefore conclude that NMES can be performed in cardiac ICD patients if 1) individual risks (e. g., pacing dependency, acute heart failure, unstable angina, ventricular arrhythmic episode in the last 3 months) are excluded by performing a safety check before starting NMES treatment and 2) “passive” exercise using NMES is performed only for thighs and gluteal muscles in 3) compliant ICD patients (especially for home-based NMES) and 4) the treatment is regularly supervised by a physician and the device is examined after the first use of NMES to exclude EMI. Nevertheless, further studies including larger sample sizes are necessary to exclude any risk when NMES is used in this patient group.
References
McMurray JJ, Adamopoulos S, Anker SD, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2013;15(3):361–2.
Valentova M, von Haehling S, Bauditz J, et al. Intestinal congestion and right ventricular dysfunction: a link with appetite loss, inflammation, and cachexia in chronic heart failure. Eur Heart J. 2016;pii(9):ehw008, Epub ahead of print.
Loncar G, Springer J, Anker M, Doehner W, Lainscak M. Cardiac cachexia: hic et nunc: “hic et nunc” – here and now. Int J Cardiol. 2015;15(201):e1–12, Epub 2015 Oct 23.
Anker SD, Sharma R. The syndrome of cardiac cachexia. Int J Cardiol. 2002;85(1):51–66.
Conraads VM, Hoymans VY, Vrints CJ. Heart failure and cachexia: insights offered from molecular biology. Front Biosci. 2008;13:325–35.
Yoshida T, Delafontaine P. Mechanisms of cachexia in chronic disease states. Am J Med Sci. 2015;350(4):250–6.
Clodi M, Säly C, Hoppichler F, Resl M, Steinwender C, Eber B. Diabetes mellitus, coronary artery disease and heart disease. Wien Klin Wochenschr. 2016;128(Suppl 2):212–5.
Von der Heidt A, Ammenwerth E, Bauer K, et al. HerzMobil Tirol network: rationale for and design of a collaborative heart failure disease management program in Austria. Wien Klin Wochenschr. 2014;126(21–22):734–41.
O’Connor CM, Whellan DJ, Lee KL, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301(14):1439–50.
Anker SD, Ponikowski P, Varney S, et al. Wasting as independent risk factor for mortality in chronic heart failure. Lancet. 1997;349(9058):1050–3.
Piepoli MF, Conraads V, Corrà U, et al. Exercise training in heart failure: from theory to practice. A consensus document of the Heart Failure Association and the European Association for Cardiovascular Prevention and Rehabilitation. Eur J Heart Fail. 2011;13(4):347–57.
Dirks ML, Hansen D, Van Assche A, Dendale P, Van Loon LJ. Neuromuscular electrical stimulation prevents muscle wasting in critically ill comatose patients. Clin Sci. 2015;128(6):357–65.
Mayr W, Bijak M, Girsch W. et al. MYOSTIM-FES to prevent muscle atrophy in microgravity and bed rest: preliminary report. Artif Organs. 1999;23(5):428–31.
Pantović M, Popović B, Madić D, Obradović J. Effects of neuromuscular electrical stimulation and resistance training on knee extensor/flexor muscles. Coll Antropol. 2015;39(Suppl 1):153–7.
Casillas JM, Gremeaux V, Labrunee M, et al. Low-frequency electromyostimulation and chronic heart failure. Ann Readapt Med Phys. 2008;51(6):461–72.
Dobsák P, Nováková M, Siegelová J, et al. Low-frequency electrical stimulation increases muscle strength and improves blood supply in patients with chronic heart failure. Circ J. 2006;70(1):75–82.
Quittan M, Sochor A, Wiesinger GF, et al. Strength improvement of knee extensor muscles in patients with chronic heart failure by neuromuscular electrical stimulation. Artif Organs. 1999;23(5):432–5.
Parissis J, Farmakis D, Karavidas A, Arapi S, Filippatos G, Lekakis J. Functional electrical stimulation of lower limb muscles as an alternative mode of exercise training in chronic heart failure: practical considerations and proposed algorithm. Eur J Heart Fail. 2015;17(12):1228–30.
Banerjee P, Caulfield B, Crowe L, Clark AL. Prolonged electrical muscle stimulation exercise improves strength, peak VO2, and exercise capacity in patients with stable chronic heart failure. J Card Fail. 2009;15(4):319–26.
Crevenna R, Posch M, Sochor A, et al. Optimizing electrotherapy-a comparative study of 3 different currents. Wien Klin Wochenschr. 2002;114(10–11):400–4.
Arena R, Pinkstaff S, Wheeler E, Peberdy MA, Guazzi M, Myers J. Neuromuscular electrical stimulation and inspiratory muscle training as potential adjunctive rehabilitation options for patients with heart failure. J Cardiopulm Rehabil Prev. 2010;30(4):209–23.
Yoshida S, Fujiwara K, Kohira S, Hirose M. Electromagnetic interference of implantable cardiac devices from a shoulder massage machine. J Artif Organs. 2014;17(3):243–9.
Binggeli C, Rickli H, Ammann P, et al. Induction ovens and electromagnetic interference: what is the risk for patients with implantable cardioverter defibrillators? J Cardiovasc Electrophysiol. 2005;16(4):399–401.
Sabaté X, Moure C, Nicolás J, Sedó M, Navarro X. Washing machine associated 50 Hz detected as ventricular fibrillation by an implanted cardioverter defibrillator. Pacing Clin Electrophysiol. 2001;24(8 Pt 1):1281–3.
McIvor ME, Reddinger J, Floden E, Sheppard RC. Study of pacemaker and implantable cardioverter defibrillator triggering by electronic article surveillance devices (SPICED TEAS). Pacing Clin Electrophysiol. 1998;21(10):1847–61.
Crevenna R, Wolzt M, Fialka-Moser V, et al. Long-term transcutaneous neuromuscular electrical stimulation in patients with bipolar sensing implantable cardioverter defibrillators: a pilot safety study. Artif Organs. 2004;28(1):99–102.
Crevenna R, Stix G, Pleiner J, et al. Electromagnetic interference by transcutaneous neuromuscular electrical stimulation in patients with bipolar sensing implantable cardioverter defibrillators: a pilot safety study. Pacing Clin Electrophysiol. 2003;26:626–9.
Nägele H, Azizi M. Inappropriate ICD discharge induced by electrical interference from a physio-therapeutic muscle stimulation device. Herzschrittmacherther Elektrophysiol. 2006;17(3):137–9.
Glotzer TV, Gordon M, Sparta M, Radoslovich G, Zimmerman J. Electromagnetic interference from a muscle stimulation device causing discharge of an implantable cardioverter defibrillator: epicardial bipolar and endocardial bipolar sensing circuits are compared. Pacing Clin Electrophysiol. 1998;21(10):1996–8.
Pyatt JR, Trenbath D, Chester M, Connelly DT. The simultaneous use of a biventricular implantable cardioverter defibrillator (ICD) and transcutaneous electrical nerve stimulation (TENS) unit: implications for device interaction. Europace. 2003;5(1):91–3.
Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377–84.
Hasenoehrl T, Keilani M, Sedghi Komanadj T, et al. The effects of resistance exercise on physical performance and health-related quality of life in prostate cancer patients: a systematic review. Support Care Cancer. 2015;23(8):2479–97.
Wayar L, Mont L, Silva RM, et al. Electrical interference from an abdominal muscle stimulator unit on an implantable cardioverter defibrillator: report of two consecutive cases. Pacing Clin Electrophysiol. 2003;26(5):1292–3.
Kamiya K, Satoh A, Niwano S, et al. Safety of neuromuscular electrical stimulation in patients implanted with cardioverter defibrillators. J Electrocardiol. 2016;49(1):99–101.
Siu CW, Tse HF, Lau CP. Inappropriate implantable cardioverter defibrillator shock from a transcutaneous muscle stimulation device therapy. J Interv Card Electrophysiol. 2005;13(1):73–5.
Vlay SC. Electromagnetic interference and ICD discharge related to chiropractic treatment. Pacing Clin Electrophysiol. 1998;21(10):2009.
Holmgren C, Carlsson T, Mannheimer C, Edvardsson N. Risk of interference from transcutaneous electrical nerve stimulation on the sensing function of implantable defibrillators. Pacing Clin Electrophysiol. 2008;31(2):151–8.
Nuhr MJ, Pette D, Berger R, et al. Beneficial effects of chronic low-frequency stimulation of thigh muscles in patients with advanced chronic heart failure. Eur Heart J. 2004;25(2):136–43.
Quittan M, Wiesinger GF, Sturm B, et al. Improvement of thigh muscles by neuromuscular electrical stimulation in patients with refractory heart failure: a single-blind, randomized, controlled trial. Am J Phys Med Rehabil. 2001;80(3):206–14, quiz 215–6, 224.
Wiesinger GF, Crevenna R, Nuhr MJ, Huelsmann M, Fialka-Moser V, Quittan M. Neuromuscular electric stimulation in heart transplantation candidates with cardiac pacemakers. Arch Phys Med Rehabil. 2001;82(10):1476–7.
Crevenna R, Quittan M, Wiesinger GF, et al. Electrical nerve stimulation in patients with cardiac pacemakers. Phys Med Rehab Kuror. 2001;11(5):159–64.
Crevenna R, Nuhr MJ, Wiesinger GF, et al. Long-term neuromuscular electrical stimulation in heart transplantation candidates with cardiac pacemakers. Phys Med Rehab Kuror. 2001;11(6):212–5.
Open access funding provided by Medical University of Vienna.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
F. Cenik, D. Schoberwalter, M. Keilani, B. Maehr, M. Wolzt, M. Marhold, and R. Crevenna declare that they have no competing interests.
Additional information
Dr. Fadime Cenik and Dr. Dieter Schoberwalter have contributed equally.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Cenik, F., Schoberwalter, D., Keilani, M. et al. Neuromuscular electrical stimulation of the thighs in cardiac patients with implantable cardioverter defibrillators. Wien Klin Wochenschr 128, 802–808 (2016). https://doi.org/10.1007/s00508-016-1045-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00508-016-1045-2