Abstract
Background
The principal objective of the experiment was to analyze the effects of the clutch operation of robotic surgical systems on the performance of the operator. The relative coordinate system introduced by the clutch operation can introduce a visual–perceptual mismatch which can potentially have negative impact on a surgeon’s performance. We also assess the impact of the introduction of additional tactile sensory information on reducing the impact of visual–perceptual mismatch on the performance of the operator.
Methods
We asked 45 novice subjects to complete peg transfers using the da Vinci IS 1200 system with grasper-mounted, normal force sensors. The task involves picking up a peg with one of the robotic arms, passing it to the other arm, and then placing it on the opposite side of the view. Subjects were divided into three groups: aligned group (no mismatch), the misaligned group (10 cm z axis mismatch), and the haptics-misaligned group (haptic feedback and z axis mismatch). Each subject performed the task five times, during which the grip force, time of completion, and number of faults were recorded.
Results
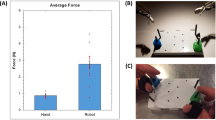
Compared to the subjects that performed the tasks using a properly aligned controller/arm configuration, subjects with a single-axis misalignment showed significantly more peg drops (p = 0.011) and longer time to completion (p < 0.001). Additionally, it was observed that addition of tactile feedback helps reduce the negative effects of visual–perceptual mismatch in some cases. Grip force data recorded from grasper-mounted sensors showed no difference between the different groups.
Conclusions
The visual–perceptual mismatch created by the misalignment of the robotic controls relative to the robotic arms has a negative impact on the operator of a robotic surgical system. Introduction of other sensory information and haptic feedback systems can help in potentially reducing this effect.





Similar content being viewed by others
References
Ghanem M, Senagore A, Shaheen S (2015) Cost and outcomes in robotic-assisted laparoscopic surgery. In: Ross H, Lee S, Champagne BJ, Pigazzi A, Rivadeneira DE (eds) Robotic approaches to colorectal surgery SE-22. Springer, Berlin, pp 267–273. doi:10.1007/978-3-319-09120-4_22
Lowrance WT, Eastham JA, Savage C et al (2012) Contemporary open and robotic radical prostatectomy practice patterns among urologists in the United States. J Urol 187(6):2087–2092. doi:10.1016/j.juro.2012.01.061
Munz Y, Moorthy K, Dosis A et al (2004) The benefits of stereoscopic vision in robotic-assisted performance on bench models. Surg Endosc 18(4):611–616. doi:10.1007/s00464-003-9017-9
Satava RM (2002) Surgical robotics: the early chronicles: a personal historical perspective. Surg Laparosc Endosc Percutan Tech 12(1):6–16. doi:10.1097/00129689-200202000-00002
Ballantyne GH (2002) Robotic surgery, telerobotic surgery, telepresence, and telementoring: review of early clinical results. Surg Endosc Other Interv Tech 16(10):1389–1402. doi:10.1007/s00464-001-8283-7
Moorthy K, Munz Y, Dosis A et al (2004) Dexterity enhancement with robotic surgery. Surg Endosc 18(5):790–795. doi:10.1007/s00464-003-8922-2
Marescaux J, Leroy J, Gagner M et al (2001) Transatlantic robot-assisted telesurgery. Nature 413(6854):379–380. doi:10.1038/35096636
Nayyar R, Gupta NP (2009) Critical appraisal of technical problems with robotic urological surgery. BJU Int 105(12):1710–1713. doi:10.1111/j.1464-410X.2009.09039.x
Bethea BT, Okamura AM, Kitagawa M et al (2004) Application of haptic feedback to robotic surgery. J Laparoendosc Adv Surg Tech A 14(3):191–195. doi:10.1089/1092642041255441
van der Meijden OA, Schijven MP (2009) The value of haptic feedback in conventional and robot-assisted minimal invasive surgery and virtual reality training: a current review. Surg Endosc 23(6):1180–1190. doi:10.1007/s00464-008-0298-x
Xin H, Zelek JS, Carnahan H (2006) Laparoscopic surgery, perceptual limitations and force: a review. First Canadian student conference on biomedical computing, Kingston, ON, pp 44–46
Fiene J, Kuchenbecker KJ, Niemeyer G (2006). Event-based haptics with grip force compensation. In: Proceedings of IEEE symposium on haptic interfaces for virtual environment and teleoperator systems, pp 117–123. http://www.stanford.edu/~katherin/pub/pdf/Kuchenbecker06-HS-Grip.pdf
Franco ML, King CH, Culjat MO et al (2009) An integrated pneumatic tactile feedback actuator array for robotic surgery. Int J Med Robot Computer Assist Surg 5:13–19. doi:10.1002/rcs.224
Kitagawa M, Dokko D, Okamura AM, Yuh DD (2005) Effect of sensory substitution on suture-manipulation forces for robotic surgical systems. J Thorac Cardiovasc Surg 129(1):151–158. doi:10.1016/j.jtcvs.2004.05.029
Kokkinara E, Slater M, López-Moliner J (2015) The effects of visuomotor calibration to the perceived space and body, through embodiment in immersive virtual reality. ACM Trans Appl Percept 13(1):1–22. doi:10.1145/2818998
Proske U, Gandevia SC (2009) The kinaesthetic senses. J Physiol 17:4139–4146. doi:10.1113/jphysiol.2009.175372
Peters JH, Fried GM, Swanstrom LL et al (2004) Development and validation of a comprehensive program of education and assessment of the basic fundamentals of laparoscopic surgery. Surgery 135(1):21–27. doi:10.1016/S0039-6060(03)00156-9
Schluender S, Conrad J, Divino CM, Gurland B (2003) Robot-assisted laparoscopic repair of ventral hernia with intracorporeal suturing: an experimental study. Surg Endosc Other Interv Tech 17(9):1391–1395. doi:10.1007/s00464-002-8795-9
Kang CM, Kim DH, Lee WJ, Chi HS (2011) Conventional laparoscopic and robot-assisted spleen-preserving pancreatectomy: does da Vinci have clinical advantages? Surg Endosc Other Interv Tech 25(6):2004–2009. doi:10.1007/s00464-010-1504-1
Beutler WJ, Peppelman WC, DiMarco LA (2013) The da Vinci robotic surgical assisted anterior lumbar interbody fusion. Spine (Phila Pa 1976) 38(4):356–363. doi:10.1097/BRS.0b013e31826b3d72
Herron DM, Marohn M (2008) A consensus document on robotic surgery. Surg Endosc 22(2):313–325. doi:10.1007/s00464-007-9727-5
Corcione F, Esposito C, Cuccurullo D et al (2005) Advantages and limits of robot-assisted laparoscopic surgery: preliminary experience. Surg Endosc Other Interv Tech 19(1):117–119. doi:10.1007/s00464-004-9004-9
Cestari A, Ferrari M, Zanoni M et al (2015) Side docking of the da Vinci robotic system for radical prostatectomy: advantages over traditional docking. J Robot Surg 9(3):243–247. doi:10.1007/s11701-015-0523-2
Hong WC, Tsai JC, Chang SD, Sorger JM (2013) Robotic skull base surgery via supraorbital keyhole approach: a cadaveric study. Neurosurgery 72(SUPPL. 1):33–38. doi:10.1227/NEU.0b013e318270d9de
Marcus HJ, Hughes-Hallett A, Cundy TP, Yang GZ, Darzi A, Nandi D (2015) da Vinci robot-assisted keyhole neurosurgery: a cadaver study on feasibility and safety. Neurosurg Rev 38(2):367–371. doi:10.1007/s10143-014-0602-2
King CH, Culjat MO, Franco ML et al (2009) Tactile feedback induces reduced grasping force in robot-assisted surgery. IEEE Trans Haptics 2:103–110. doi:10.1109/TOH.2009.4
Culjat MO, King C-H, Franco ML et al (2008) A tactile feedback system for robotic surgery. Conf Proc IEEE Eng Med Biol Soc 2008:1930–1934. doi:10.1109/IEMBS.2008.4649565
Acknowledgements
This research was partially supported by the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under award number R01 EB019473 01. We thank our colleagues who provided insight and expertise that greatly assisted the research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Dr. Erik P. Duston is on Scientific Advisory board of Titan Medical. Ahmad Abiri, Anna Tao, Meg LaRocca, Xingmin Guan, Syed J. Askari, James W. Bisley, and Warren S. Grundfest have no conflicts of interest or financial ties to disclose.
Rights and permissions
About this article
Cite this article
Abiri, A., Tao, A., LaRocca, M. et al. Visual–perceptual mismatch in robotic surgery. Surg Endosc 31, 3271–3278 (2017). https://doi.org/10.1007/s00464-016-5358-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-016-5358-z