Abstract
The purpose of this study was to develop and utilize semi-quantitative RT-PCR and PCR assays for measuring the level of Cryspovirus, the viral symbiont of Cryptosporidium parvum, during in vitro development of the protozoan. Cultures of human carcinoma cells (HCT-8) were inoculated with excysting C. parvum sporozoites, followed by harvest of cells and culture medium at 2-, 24-, 48-, and 72-h post-infection. Changes in viral RNA levels were detected by RT-PCR using primers specific for RNA encoding the 40-kDa capsid protein (CP) or RNA-dependent RNA polymerase (RdRp). Parasite or host DNA was quantified by PCR specific for C. parvum or human glyceraldehyde-3-phosphate dehydrogenase (HuGAPDH). An internal standard (competitor) was incorporated into all assays as a control for PCR inhibition. Intracellular levels of C. parvum DNA increased between 2- and 48-h post-infection, and then decreased at 72 h. Culture medium overlying these C. parvum-infected cells displayed a similar increase in CP and RdRp signal, reaching peak levels at 48 h. However, the CP and RdRp levels in cellular RNA displayed only a modest increase between 2 and 48 h, and exhibited no change (CP) or decreased (RdRp) at 72 h. These data suggest that during the first 48 h of C. parvum in vitro development, Cryspovirus is released into the media overlying cells but remains at fairly constant levels within infected cells.




Similar content being viewed by others
References
Abrahamsen MS, Templeton TJ, Enomoto S, Abrahante JE, Zhu G, Lancto CA, Deng M, Liu C, Widmer G, Tzipori S, Buck GA, Xu P, Bankier AT, Dear PH, Konfortov BA, Spriggs HF, Iyer V, Anantharaman A, Kapur V (2004) Complete genome sequence of the apicomplexan, Cryptosporidium parvum. Science 304:441–445
Arai T, Kimata I, Kitade Y, Nakamoto K, Tokoro M (2011) In vitro assessment of anticryptosporidial efficacy and cytotoxicity of adenosine analogues using a SYBR Green real-time PCR method. J Antimicrob Chemother 66:560–563
Banik GR, Stark D, Rashid H, Ellis JT (2014) Recent advances in molecular biology of parasitic viruses. Infect Disord Drug Targets 2014 Jul 13. [Epub ahead of print].
Fichorova RN, Lee Y, Yamamoto HS, Takagi Y, Hayes GR, Goodman RP, Chepa-Lotrea X, Buck OR, Murray R, Kula T, Beach DH, Singh BN, Nibert ML (2012) Endobiont viruses sensed by the human host—beyond conventional antiparasitic therapy. PLoS One 7(11):e48418
Hanahan D (1983) Studies on the transformation of Escherichia coli with plasmids. J Mol Biol 166:557–580
Hartley MA, Ronet C, Zangger H, Beverley SM, Fasel N (2012) Leishmania RNA virus: when the host pays the toll. Front Cell Infect Microbiol 2:99–105
Hommer V, Eichholz J, Petry F (2003) Effect of antiretroviral protease inhibitors alone, and in combination with paromomycin, on the excystation, invasion and in vitro development of Cryptosporidium parvum. J Antimicrob Chemother 52:359–364
Jakobi V, Petry F (2006) Differential expression of Cryptosporidium parvum genes encoding sporozoite surface antigens in infected HCT-8 host cells. Microbes Infect 8:2186–2194
Jenkins MC, O’Brien C, Trout J, Guidry A, Fayer R (1998) Hyperimmune bovine colostrum specific for recombinant Cryptosporidium parvum antigen confers partial protection against cryptosporidiosis in immunosuppressed adult mice. Vaccine 17:2453–2460
Jenkins MC, Higgins J, Abrahante JE, Kniel KE, O’Brien C, Trout J, Lancto CA, Abrahamsen MS, Fayer R (2007) Fecundity of Cryptosporidium parvum is correlated with intracellular levels of the viral symbiont CPV. Int J Parasitol 38:1051–1055
Joachim A, Eckert E, Petry F, Bialek R, Daugschies A (2003) Comparison of viability assays for Cryptosporidium parvum oocysts after disinfection. Vet Parasitol 111:47–57
Khramtsov NV, Upton SJ (1998) High-temperature inducible cell-free transcription and replication of double-stranded RNAs within the parasitic protozoan Cryptosporidium parvum. Virology 245:331–337
Khramtsov NV, Woods KM, Nesterenko MV, Dykstra CC, Upton SJ (1997) Virus-like, double-stranded RNAs in the parasitic protozoan Cryptosporidium parvum. Mol Microbiol 26:289–300
Khramtsov NV, Chung PA, Dykstra CC, Griffiths JK, Morgan UM, Arrowood MJ, Upton SJ (2000) Presence of double-stranded RNAs in human and calf isolates of Cryptosporidium parvum. J Parasitol 86:275–282
Lemgruber L, Lupetti P (2012) Crystalloid body, refractile body and virus-like particles in Apicomplexa: what is in there? Parasitology 139:285–293
Mele R, Gomez-Morales MA, Tosini F, Pozio E (2004) Cryptosporidium parvum at different developmental stages modulates host cell apoptosis in vitro. Infect Immun 72:6061–6067
Miller RL, Wang AL, Wang CC (1988) Purification and characterization of the Giardia lamblia double-stranded RNA virus. Mol Biochem Parasitol 28:189–195
Najdrowski M, Joachim A, Daugschies A (2007) An improved in vitro infection model for viability testing of Cryptosporidium parvum oocysts. Vet Parasitol 150:150–154
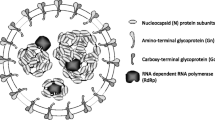
Nibert ML, Woods KM, Upton SJ, Ghabrial SA (2009) Cryspovirus: a new genus of protozoan viruses in the family Partitiviridae. Arch Virol 154:1959–1965
Nibert ML, Ghabrial SA, Maiss E, Lesker T, Vainio EJ, Jiang D, Suzuki N (2014) Taxonomic reorganization of family Partitiviridae and other recent progress in partitivirus research. Virus Res 188:128–141
Ross R, Kleinz R, Reske-Kunz AB (1995) A method for rapid generation of competitive standard molecules for RT-PCR avoiding the problem of competitor/probe cross-reactions. PCR Methods Appl 4:371–375
Shahiduzzaman M, Dyachenko V, Obwaller A, Unglaube S, Daugschies A (2009) Combination of cell culture and quantitative PCR for screening of drugs against Cryptosporidium parvum. Vet Parasitol 162:271–277
Shahiduzzaman M, Dyachenko V, Keidel J, Schmäschke R, Daugschies A (2010) Combination of cell culture and quantitative PCR (cc-qPCR) to assess disinfectants efficacy on Cryptosporidium oocysts under standardized conditions. Vet Parasitol 167:43–49
Teichmann K, Kuliberda M, Schatzmayr G, Hadacek F, Joachim A (2012) In vitro determination of anticryptosporidial activity of phytogenic extracts and compounds. Parasitol Res 111:231–240
Upton SJ, Tilley M, Brillhart DB (1995) Effects of select medium supplements on in vitro development of Cryptosporidium parvum in HCT-8 cells. J Clin Microbiol 33:371–375
Xiao L, Limor J, Bern C, Lal AA (2001) Tracking Cryptosporidium parvum by sequence analysis of small double-stranded RNA. Emerg Infect Dis 7:141–145
Acknowledgments
Funding for this research was provided under USDA-ARS CRIS Project No. 1245-32000-089-00D.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jenkins, M.C., O’Brien, C.N., Santin, M. et al. Changes in the levels of Cryspovirus during in vitro development of Cryptosporidium parvum . Parasitol Res 114, 2063–2068 (2015). https://doi.org/10.1007/s00436-015-4390-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-015-4390-6