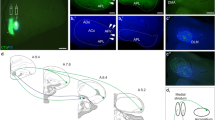
Abstract
We previously analyzed the arborization patterns of rat ventral pallidal (VP) axons that coursed caudally to innervate the thalamus and brainstem (Tripathi et al. in Brain Struct Funct 218:1133–1157, 2013). Here, we have reconstructed 16 previously undetected axons from the same tracer deposits that follow a more lateral trajectory. Virtually all 16 axons emanating from the different VP compartments collateralized in the extended amygdala system (EAS) and amygdaloid complex. The most frequent targets of axons from the lateral and medial (VPm) VP compartments were the rostral sublenticular extended amygdala, the extended amygdala (EA), the central nucleus of the amygdala and the posterior part of the basolateral amygdaloid nucleus. In contrast, axons from the rostral extension of the VP preferentially innervated the anterior amygdaloid area, the magnocellular preoptic nucleus, and the anterior part of the basomedial amygdaloid nucleus. We additionally found and reconstructed a single corticopetal axon arising from the VPm. The new results show that both direct and indirect projections from the basolateral complex and EAS to the ventral striatopallidal system are reciprocated by VP projections, and suggest that the systems can be activated simultaneously. The results additionally suggest that the amygdaloid complex and cortex are innervated separately from the VP. Finally, the combination of new and previous data indicate that approximately 84 % of VP axons (88/105) participate in basal ganglia circuits, 15 % (16/105) target the amygdaloid complex, and less than 1 % innervate the cortex.










Similar content being viewed by others
Abbreviations
- AA:
-
Anterior amygdaloid area
- acp:
-
Anterior commissure posterior part
- Acb:
-
Nucleus accumbens
- AcbC:
-
Nucleus accumbens, core
- AcbSh:
-
Nucleus accumbens, shell
- AHi:
-
Amygdalohippocampal area
- AHiAL:
-
Amygdalohippocampal area, anterolateral part
- AHiPM:
-
Amygdalohippocampal area, posteromedial part
- APir:
-
Amygdalopiriform transition area
- B:
-
Basal nucleus of Meynert
- BDA:
-
Biotinylated dextran amine
- BL:
-
Basolateral amygdaloid nucleus
- BLA:
-
Basolateral amygdaloid nucleus, anterior part
- BLP:
-
Basolateral amygdaloid nucleus, posterior part
- BLV:
-
Basolateral amygdaloid nucleus, ventral part
- BM:
-
Basomedial amygdaloid nucleus
- BMA:
-
Basomedial amygdaloid nucleus, anterior part
- BMP:
-
Basomedial amygdaloid nucleus, posterior part
- BST:
-
Bed nucleus of the stria terminalis
- BSTL:
-
Bed nucleus of the stria terminalis, lateral division
- BSTM:
-
Bed nucleus of the stria terminalis, medial division
- C:
-
Caudal
- CalB:
-
Calbindin D28k
- Ce:
-
Central amygdaloid nucleus
- CeC:
-
Central amygdala nucleus, capsular
- CeL:
-
Central amygdala nucleus, lateral
- CeM:
-
Central amygdala nucleus, medial
- CPu:
-
Caudate putamen
- cst:
-
Commissural stria terminalis
- CxA:
-
Cortex–amygdala transition zone
- D:
-
Dorsal
- DP:
-
Dorsal peduncular cortex
- DTT:
-
Dorsal tenia tecta
- EA:
-
Extended amygdala
- EAS:
-
Extended amygdala system
- Enk:
-
Leu-enkephalin
- EP:
-
Entopeduncular nucleus
- f:
-
Fornix
- FrA:
-
Frontal association cortex
- GP:
-
Globus pallidus
- I:
-
Intercalated nuclei of amygdala
- ic:
-
Internal capsule
- IL:
-
Infralimbic cortex
- IPAC:
-
Interstitial nucleus of posterior limb of anterior commissure
- L:
-
Lateral
- LaDL:
-
Lateral amygdaloid nucleus, dorsolateral
- LaVM:
-
Lateral amygdaloid nucleus, ventromedial
- LHb:
-
Lateral habenula
- LSS:
-
Lateral stripe of striatum
- LV:
-
Lateral ventricle
- MCPO:
-
Magnocellular preoptic nucleus
- Me:
-
Medial amygdaloid nucleus
- MeAD:
-
Medial amygdaloid nucleus, anterodorsal
- MeAV:
-
Medial amygdaloid nucleus, anteroventral
- MePD:
-
Medial amygdaloid nucleus, posterodorsal
- MePV:
-
Medial amygdaloid nucleus, posteroventral
- opt:
-
Optic tract
- Pir:
-
Piriform cortex
- PLCo:
-
Posterolateral cortical amygdaloid nucleus
- PMCo:
-
Posteromedial cortical amygdaloid nucleus
- PrL:
-
Prelimbic cortex
- Sib :
-
Substantia innominata, basal part
- SLEA:
-
Sublenticular extended amygdala
- SLEAr:
-
Rostral sublenticular extended amygdala
- STIA:
-
Bed nucleus of stria terminalis intraamygdaloid division
- Tu:
-
Olfactory tubercle
- VEn:
-
Ventral endopiriform nucleus
- VP:
-
Ventral pallidum
- VPl:
-
Ventral pallidum, dorsolateral compartment
- VPm:
-
Ventral pallidum, ventromedial compartment
- VPr:
-
Ventral pallidum, rostral portion
References
Abdi A, Mallet N, Mohamed FY, Sharott A, Dodson PD, Nakamura KC, Suri S, Avery SV, Larvin JT, Garas FN, Garas SN, Vinciati F, Morin S, Bezard E, Baufreton J, Magill PJ (2015) Prototypic and arkypallidal neurons in the dopamine-intact external globus pallidus. J Neurosci 35:6667–6688
Alheid GF, Heimer L (1988) New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience 27:1–39
Anglada-Figueroa D, Quirk GJ (2005) Lesions of the basal amygdala block expression of conditioned fear but not extinction. J Neurosci 25:9680–9685
Balleine BW, Killcross S (2006) Parallel incentive processing: an integrated view of amygdala function. Trends Neurosci 29:272–279
Baufreton J, Kirkham E, Atherton JF, Menard A, Magill PJ, Bolam JP, Bevan MD (2009) Sparse but selective and potent synaptic transmission from the globus pallidus to the subthalamic nucleus. J Neurophysiol 102:532–545
Berendse HW, Galis-de Graaf Y, Groenewegen HJ (1992) Topographical organization and relationship with ventral striatal compartments of prefrontal corticostriatal projections in the rat. J Comp Neurol 316:314–347
Bernard JF, Alden M, Besson JM (1993) The organization of the efferent projections from the pontine parabrachial area to the amygdaloid complex: a Phaseolus vulgaris leucoagglutinin (PHA-L) study in the rat. J Comp Neurol 329:201–229
Bienkowski MS, Rinaman L (2012) Common and distinct neural inputs to the medial central nucleus of the amygdala and anterior ventrolateral bed nucleus of stria terminalis in rats. Brain Struct Funct 218:187–208
Bigl V, Woolf NJ, Butcher LL (1982) Cholinergic projections from the basal forebrain to frontal, parietal, temporal, occipital, and cingulate cortices: a combined fluorescent tracer and acetylcholinesterase analysis. Brain Res Bull 8:727–749
Bolam JP, Ingham CA, Izzo PN, Levey AI, Rye DB, Smith AD, Wainer BH (1986) Substance P-containing terminals in synaptic contact with cholinergic neurons in the neostriatum and basal forebrain: a double immunocytochemical study in the rat. Brain Res 397:279–289
Canteras NS, Simerly RB, Swanson LW (1995) Organization of projections from the medial nucleus of the amygdala: a PHAL study in the rat. J Comp Neurol 360:213–245
Cardinal RN, Parkinson JA, Hall J, Everitt BJ (2002) Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev 26:321–352
Carlsen J, Zaborszky L, Heimer L (1985) Cholinergic projections from the basal forebrain to the basolateral amygdaloid complex: a combined retrograde fluorescent and immunohistochemical study. J Comp Neurol 234:155–167
Cebrián C, Parent A, Prensa L (2005) Patterns of axonal branching of neurons of the substantia nigra pars reticulata and pars lateralis in the rat. J Comp Neurol 492:349–369
Ciocchi S, Herry C, Grenier F, Wolff SB, Letzkus JJ, Vlachos I, Ehrlich I, Sprengel R, Deisseroth K, Stadler MB, Muller C, Luthi A (2010) Encoding of conditioned fear in central amygdala inhibitory circuits. Nature 468:277–282
Commons KG, Beck SG, Bey VW (2005) Two populations of glutamatergic axons in the rat dorsal raphe nucleus defined by the vesicular glutamate transporters 1 and 2. Eur J Neurosci 21:1577–1586
Comoli E, Ribeiro-Barbosa ER, Negrao N, Goto M, Canteras NS (2005) Functional mapping of the prosencephalic systems involved in organizing predatory behavior in rats. Neuroscience 130:1055–1067
de Olmos JS, Heimer L (1999) The concepts of the ventral striatopallidal system and extended amygdala. Ann N Y Acad Sci 877:1–32
de Olmos JS, Beltramino CA, Alheid G (2004) Amygdala and extended amygdala of the rat: a cytoarchitectonical, fibroarchitectonical, and chemoarchitectonical survey. In: Paxinos G (ed) The rat nervous system, 3rd edn. Elsevier, Oxford, pp 509–603
Divac I (1975) Magnocellular nuclei of the basal forebrain project to neocortex, brain stem, and olfactory bulb. Review of some functional correlates. Brain Res 93:385–398
Duque A, Tepper JM, Detari L, Ascoli GA, Zaborszky L (2007) Morphological characterization of electrophysiologically and immunohistochemically identified basal forebrain cholinergic and neuropeptide Y-containing neurons. Brain Struct Funct 212:55–73
Everitt BJ, Robbins TW (1992) Amygdala-ventral striatal interactions and reward-related processes. In: Aggleton JP (ed) The amygdala. Wiley, New York, pp 401–430
Fox AS, Oler JA, Tromp do PM, Fudge JL, Kalin NH (2015) Extending the amygdala in theories of threat processing. Trends Neurosci 38:319–329
Furuta T, Kaneko T (2006) Third pathway in the cortico-basal ganglia loop: Neurokinin B-producing striatal neurons modulate cortical activity via striato-innominato-cortical projection. Neurosci Res 54:1–10
Furuta T, Mori T, Lee T, Kaneko T (2000) Third group of neostriatofugal neurons: neurokinin B-producing neurons that send axons predominantly to the substantia innominata. J Comp Neurol 426:279–296
Furuta T, Zhou L, Kaneko T (2002) Preprodynorphin-, preproenkephalin-, preprotachykinin A- and preprotachykinin B-immunoreactive neurons in the accumbens nucleus and olfactory tubercle: double-immunofluorescence analysis. Neuroscience 114:611–627
Furuta T, Koyano K, Tomioka R, Yanagawa Y, Kaneko T (2004) GABAergic basal forebrain neurons that express receptor for neurokinin B and send axons to the cerebral cortex. J Comp Neurol 473:43–58
Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ (2005) Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol 492:145–177
Gittis AH, Berke JD, Bevan MD, Chan CS, Mallet N, Morrow MM, Schmidt R (2014) New roles for the external globus pallidus in Basal Ganglia circuits and behavior. J Neurosci 34:15178–15183
Gritti I, Mainville L, Mancia M, Jones BE (1997) GABAergic and other noncholinergic basal forebrain neurons, together with cholinergic neurons, project to the mesocortex and isocortex in the rat. J Comp Neurol 383:163–177
Groenewegen HJ, Becker NE, Lohman AH (1980) Subcortical afferents of the nucleus accumbens septi in the cat, studied with retrograde axonal transport of horseradish peroxidase and bisbenzimid. Neuroscience 5:1903–1916
Grove EA (1988a) Efferent connections of the substantia innominata in the rat. J Comp Neurol 277:347–364
Grove EA (1988b) Neural associations of the substantia innominata in the rat: afferent connections. J Comp Neurol 277:315–346
Grove EA, Domesick VB, Nauta WJ (1986) Light microscopic evidence of striatal input to intrapallidal neurons of cholinergic cell group Ch4 in the rat: a study employing the anterograde tracer Phaseolus vulgaris leucoagglutinin (PHA-L). Brain Res 367:379–384
Haber SN, Knutson B (2010) The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 35:4–26
Haber S, McFarland NR (2001) The place of the thalamus in frontal cortical-basal ganglia circuits. Neuroscientist 7:315–324
Haber SN, Nauta WJ (1983) Ramifications of the globus pallidus in the rat as indicated by patterns of immunohistochemistry. Neuroscience 9:245–260
Haber SN, Groenewegen HJ, Grove EA, Nauta WJ (1985) Efferent connections of the ventral pallidum: evidence of a dual striato pallidofugal pathway. J Comp Neurol 235:322–335
Heimer L, Van Hoesen GW (2006) The limbic lobe and its output channels: implications for emotional functions and adaptive behavior. Neurosci Biobehav Rev 30:126–147
Heimer L, Wilson RD (1975) The subcortical projections of the allocortex: similarities in the neural associations of the hippocampus, the piriform cortex, and the neocortex. In: Santini M (ed) Golgi centennial symposium. Proceedings. Raven Press, New York, pp 177–193
Heimer L, Zaborszky L, Zahm DS, Alheid GF (1987) The ventral striatopallidothalamic projection: I. The striatopallidal link originating in the striatal parts of the olfactory tubercle. J Comp Neurol 255:571–591
Heimer L, Zahm DS, Churchill L, Kalivas PW, Wohltmann C (1991) Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience 41:89–125
Heimer L, Harlan RE, Alheid GF, Garcia MM, de Olmos J (1997) Substantia innominata: a notion which impedes clinical-anatomical correlations in neuropsychiatric disorders. Neuroscience 76:957–1006
Henderson Z (1997) The projection from the striatum to the nucleus basalis in the rat: an electron microscopic study. Neuroscience 78:943–955
Hikosaka O, Bromberg-Martin E, Hong S, Matsumoto M (2008) New insights on the subcortical representation of reward. Curr Opin Neurobiol 18:203–208
Jacobsohn L (1909) Über die Kerne des menschlichen hirnstamms. Verlag der Königl Akademie des Wisenschaftern, Berlin
Kelley AE, Domesick VB, Nauta WJ (1982) The amygdalostriatal projection in the rat—an anatomical study by anterograde and retrograde tracing methods. Neuroscience 7:615–630
Krettek JE, Price JL (1978) Amygdaloid projections to subcortical structures within the basal forebrain and brainstem in the rat and cat. J Comp Neurol 178:225–254
Liu AK, Chang RC, Pearce RK, Gentleman SM (2015) Nucleus basalis of Meynert revisited: anatomy, history and differential involvement in Alzheimer’s and Parkinson’s disease. Acta Neuropathol 129:527–540
Mallet N, Micklem BR, Henny P, Brown MT, Williams C, Bolam JP, Nakamura KC, Magill PJ (2012) Dichotomous organization of the external globus pallidus. Neuron 74:1075–1086
Martinez-Garcia F, Novejarque A, Lanuza E (2008) Two interconnected functional systems in the amygdala of amniote vertebrates. Brain Res Bull 75:206–213
Mascagni F, McDonald AJ (2009) Parvalbumin-immunoreactive neurons and GABAergic neurons of the basal forebrain project to the rat basolateral amygdala. Neuroscience 160:805–812
McDonald AJ, Shammah-Lagnado SJ, Shi C, Davis M (1999) Cortical afferents to the extended amygdala. Ann N Y Acad Sci 877:309–338
McDonald AJ, Muller JF, Mascagni F (2011) Postsynaptic targets of GABAergic basal forebrain projections to the basolateral amygdala. Neuroscience 183:144–159
McFarland NR, Haber SN (2002) Thalamic relay nuclei of the basal ganglia form both reciprocal and nonreciprocal cortical connections, linking multiple frontal cortical areas. J Neurosci 22:8117–8132
McGinty VB, Hayden BY, Heilbronner SR, Dumont EC, Graves SM, Mirrione MM, du Hoffmann J, Sartor GC, Espana RA, Millan EZ, Difeliceantonio AG, Marchant NJ, Napier TC, Root DH, Borgland SL, Treadway MT, Floresco SB, McGinty JF, Haber S (2011) Emerging, reemerging, and forgotten brain areas of the reward circuit: notes from the 2010 motivational neural networks conference. Behav Brain Res 225:348–357
Mello LE, Tan AM, Finch DM (1992) GABAergic synaptic transmission in projections from the basal forebrain and hippocampal formation to the amygdala: an in vivo iontophoretic study. Brain Res 587:41–48
Mesulam MM, Mufson EJ, Wainer BH, Levey AI (1983) Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1–Ch6). Neuroscience 10:1185–1201
Newman SW (1999) The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann N Y Acad Sci 877:242–257
Nobrega-Pereira S, Gelman D, Bartolini G, Pla R, Pierani A, Marin O (2010) Origin and molecular specification of globus pallidus neurons. J Neurosci 30:2824–2834
Novejarque A, Gutierrez-Castellanos N, Lanuza E, Martinez-Garcia F (2011) Amygdaloid projections to the ventral striatum in mice: direct and indirect chemosensory inputs to the brain reward system. Front Neuroanat 5:54
Ottersen OP (1980) Afferent connections to the amygdaloid complex of the rat and cat: II. Afferents from the hypothalamus and the basal telencephalon. J Comp Neurol 194:267–289
Ottersen OP (1982) Connections of the amygdala of the rat. IV: corticoamygdaloid and intraamygdaloid connections as studied with axonal transport of horseradish peroxidase. J Comp Neurol 205:30–48
Palomero-Gallagher N, Zilles K (2004) Isocortex. In: Paxinos G (ed) The rat nervous system, 3rd edn. Elsevier, Oxford, pp 729–747
Paxinos G, Watson C (1998) The rat brain in stereotaxic coordinates. Academic Press, CA
Paxinos G, Watson C (2007) The rat brain in stereotaxic coordinates. Academic Press, San Diego
Pro-Sistiaga P, Mohedano-Moriano A, Ubeda-Banon I, Del Mar Arroyo-Jimenez M, Marcos P, Artacho-Perula E, Crespo C, Insausti R, Martinez-Marcos A (2007) Convergence of olfactory and vomeronasal projections in the rat basal telencephalon. J Comp Neurol 504:346–362
Rao ZR, Shiosaka S, Tohyama M (1987) Origin of cholinergic fibers in the basolateral nucleus of the amygdaloid complex by using sensitive double-labeling technique of retrograde biotinized tracer and immunocytochemistry. J Hirnforsch 28:553–560
Rasband, W.S. (1997-2012) ImageJ. US National Institutes of Health, Bethesda. http://imagej.nih.gov/ij/
Root DH, Melendez RI, Zaborszky L, Napier TC (2015) The ventral pallidum: subregion-specific functional anatomy and roles in motivated behaviors. Prog Neurobiol 130:29–70
Russchen FT (1982) Amygdalopetal projections in the cat. II. Subcortical afferent connections. A study with retrograde tracing techniques. J Comp Neurol 207:157–176
Russchen FT, Price JL (1984) Amygdalostriatal projections in the rat. Topographical organization and fiber morphology shown using the lectin PHA-L as an anterograde tracer. Neurosci Lett 47:15–22
Rye DB, Wainer BH, Mesulam MM, Mufson EJ, Saper CB (1984) Cortical projections arising from the basal forebrain: a study of cholinergic and noncholinergic components employing combined retrograde tracing and immunohistochemical localization of choline acetyltransferase. Neuroscience 13:627–643
Saper CB (1984) Organization of cerebral cortical afferent systems in the rat. II. Magnocellular basal nucleus. J Comp Neurol 222:313–342
Sarter M, Bruno JP (2002) The neglected constituent of the basal forebrain corticopetal projection system: GABAergic projections. Eur J Neurosci 15:1867–1873
Sarter M, Bruno JP, Givens B (2003) Attentional functions of cortical cholinergic inputs: What does it mean for learning and memory? Neurobiol Learn Mem 80:245–256
Saunders A, Granger AJ, Sabatini BL (2015a) Corelease of acetylcholine and GABA from cholinergic forebrain neurons. Elife 4. doi:10.7554/eLife.06412
Saunders A, Oldenburg IA, Berezovskii VK, Johnson CA, Kingery ND, Elliott HL, Xie T, Gerfen CR, Sabatini BL (2015b) A direct GABAergic output from the basal ganglia to frontal cortex. Nature 521:85–89
Scalia F, Winans SS (1975) The differential projections of the olfactory bulb and accessory olfactory bulb in mammals. J Comp Neurol 161:31–55
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682
Sesack SR, Grace AA (2010) Cortico-Basal Ganglia reward network: microcircuitry. Neuropsychopharmacology 35:27–47
Shammah-Lagnado SJ, Alheid GF, Heimer L (1999) Afferent connections of the interstitial nucleus of the posterior limb of the anterior commissure and adjacent amygdalostriatal transition area in the rat. Neuroscience 94:1097–1123
Shammah-Lagnado SJ, Alheid GF, Heimer L (2001) Striatal and central extended amygdala parts of the interstitial nucleus of the posterior limb of the anterior commissure: evidence from tract-tracing techniques in the rat. J Comp Neurol 439:104–126
Shipley MT, Ennis M, Puche AC (2004) Olfactory System. In: Paxinos G (ed) The rat nervous system, 3rd edn. Elsevier, Oxford, pp 923–964
Stefanik MT, Kupchik YM, Brown RM, Kalivas PW (2013) Optogenetic evidence that pallidal projections, not nigral projections, from the nucleus accumbens core are necessary for reinstating cocaine seeking. J Neurosci 33:13654–13662
Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, Tye KM, Kempadoo KA, Zhang F, Deisseroth K, Bonci A (2011) Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature 475:377–380
Tripathi A, Mengual E (2012) Axonal collateralization patterns of the rostral sublenticular extended amygdala in the rat. FENS Forum 2012, Barcelona
Tripathi A, Prensa L, Cebrian C, Mengual E (2010) Axonal branching patterns of nucleus accumbens neurons in the rat. J Comp Neurol 518:4649–4673
Tripathi A, Prensa L, Mengual E (2013) Axonal branching patterns of ventral pallidal neurons in the rat. Brain Struct Funct 218:1133–1157
Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, Thompson KR, Gradinaru V, Ramakrishnan C, Deisseroth K (2011) Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature 471:358–362
Vertes RP (2004) Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse 51:32–58
Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE (2006) Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J Neurosci 26:12387–12396
Woolf NJ, Butcher LL (1982) Cholinergic projections to the basolateral amygdala: a combined Evans blue and acetylcholinesterase analysis. Brain Res Bull 8:751–763
Woolf NJ, Eckenstein F, Butcher LL (1983) Cholinergic projections from the basal forebrain to the frontal cortex: a combined fluorescent tracer and immunohistochemical analysis in the rat. Neurosci Lett 40:93–98
Woolf NJ, Eckenstein F, Butcher LL (1984) Cholinergic systems in the rat brain: I. Projections to the limbic telencephalon. Brain Res Bull 13:751–784
Yasui Y, Breder CD, Saper CB, Cechetto DF (1991) Autonomic responses and efferent pathways from the insular cortex in the rat. J Comp Neurol 303:355–374
Zaborszky L, Cullinan WE (1992) Projections from the nucleus accumbens to cholinergic neurons of the ventral pallidum: a correlated light and electron microscopic double-immunolabeling study in rat. Brain Res 570:92–101
Zaborszky L, Heimer L, Eckenstein F, Leranth C (1986) GABAergic input to cholinergic forebrain neurons: an ultrastructural study using retrograde tracing of HRP and double immunolabeling. J Comp Neurol 250:282–295
Zaborszky L, Hoemke L, Mohlberg H, Schleicher A, Amunts K, Zilles K (2008) Stereotaxic probabilistic maps of the magnocellular cell groups in human basal forebrain. Neuroimage 42:1127–1141
Zaborszky L, Csordas A, Mosca K, Kim J, Gielow MR, Vadasz C, Nadasdy Z (2015) Neurons in the basal forebrain project to the cortex in a complex topographic organization that reflects corticocortical connectivity patterns: an experimental study based on retrograde tracing and 3D reconstruction. Cereb Cortex 25:118–137
Zahm DS (1989) The ventral striatopallidal parts of the basal ganglia in the rat–II. Compartmentation of ventral pallidal efferents. Neuroscience 30:33–50
Zahm DS (2006) The evolving theory of basal forebrain functional-anatomical ‘macrosystems’. Neurosci Biobehav Rev 30:148–172
Zahm DS, Heimer L (1990) Two transpallidal pathways originating in the rat nucleus accumbens. J Comp Neurol 302:437–446
Zahm DS, Heimer L (1993) Specificity in the efferent projections of the nucleus-accumbens in the rat—comparison of the rostral pole projection patterns with those of the core and shell. J Comp Neurol 327:220–232
Zahm DS, Williams E, Wohltmann C (1996) Ventral striatopallidothalamic projection: IV. Relative involvements of neurochemically distinct subterritories in the ventral pallidum and adjacent parts of the rostroventral forebrain. J Comp Neurol 364:340–362
Zhou L, Furuta T, Kaneko T (2004) Neurokinin B-producing projection neurons in the lateral stripe of the striatum and cell clusters of the accumbens nucleus in the rat. J Comp Neurol 480:143–161
Acknowledgments
We want to thank John F. Wesseling for critical readings of the manuscript. S. Mongia is recipient of a predoctoral Grant from Asociación de Amigos de la Universidad de Navarra, while A. Tripathi was recipient of a predoctoral Grant from FIMA (Foundation for Applied Medical Research). This work was supported by the Spanish Ministry of Education and Science (MEC, BFU2004-06825), Gobierno de Navarra 2004, and the ‘UTE project CIMA’.
Author information
Authors and Affiliations
Corresponding author
Additional information
S. Mongia and A. Tripathi have contributed equally to this paper.
Rights and permissions
About this article
Cite this article
Mongia, S., Tripathi, A. & Mengual, E. Arborization patterns of amygdalopetal axons from the rat ventral pallidum. Brain Struct Funct 221, 4549–4573 (2016). https://doi.org/10.1007/s00429-016-1184-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-016-1184-2