Abstract
Purpose
We determined the effect of habitual endurance exercise and age on aortic pulse wave velocity (aPWV), augmentation pressure (AP) and systolic blood pressure (aSBP), with statistical adjustments of aPWV and AP for heart rate and aortic mean arterial pressure, when appropriate. Furthermore, we assessed whether muscle sympathetic nerve activity (MSNA) correlates with AP in young and middle-aged men.
Methods
Aortic PWV, AP, aortic blood pressure (applanation tonometry; SphygmoCor) and MSNA (peroneal microneurography) were recorded in 46 normotensive men who were either young or middle-aged and endurance-trained runners or recreationally active nonrunners (10 nonrunners and 13 runners within each age-group). Between-group differences and relationships between variables were assessed via ANOVA/ANCOVA and Pearson product-moment correlation coefficients, respectively.
Results
Adjusted aPWV and adjusted AP were similar between runners and nonrunners in both age groups (all, P > 0.05), but higher with age (all, P < 0.001), with a greater effect size for the age-related difference in AP in runners (Hedges’ g, 3.6 vs 2.6). aSBP was lower in young (P = 0.009; g = 2.6), but not middle-aged (P = 0.341; g = 1.1), runners compared to nonrunners. MSNA burst frequency did not correlate with AP in either age group (young: r = 0.00, P = 0.994; middle-aged: r = − 0.11, P = 0.604).
Conclusion
There is an age-dependent effect of habitual exercise on aortic haemodynamics, with lower aSBP in young runners compared to nonrunners only. Statistical adjustment of aPWV and AP markedly influenced the outcomes of this study, highlighting the importance of performing these analyses. Further, peripheral sympathetic vasomotor outflow and AP were not correlated in young or middle-aged normotensive men.
Similar content being viewed by others
Introduction
Aortic augmentation pressure (AP) represents a manifestation that presents in mid-systole due to the interaction between forward and backward travelling pressure waves, aortic stiffness (i.e. aortic pulse wave velocity, aPWV), and aortic reservoir pressure (O'Rourke and Mancia 1999; Mynard et al. 2020). Notably, AP and aPWV increase with age (McEniery et al. 2005) and contribute to the increase aortic systolic blood pressure (aSBP), which is predictive of future cardiovascular events in the general population (Lamarche et al. 2020). Whether lifestyle factors, such as habitual endurance exercise, influence AP, aPWV, and aSBP similarly is currently unclear.
As recently highlighted by Van Bortel et al. (2020), erroneous conclusions can be made when comparing aortic stiffness between individuals without the appropriate statistical adjustment of aPWV for mean arterial pressure (MAP). Furthermore, it is well known that habitual endurance exercise lowers heart rate (Katona et al. 1982) and that heart rate influences both AP and aPWV (Wilkinson et al. 2000, 2002; Tan et al. 2016). Therefore, in the presence of between-group differences in MAP and/or heart rate, statistical adjustments for these confounders should be made (Stoner et al. 2014). In the studies conducted to date comparing aPWV between endurance-trained and untrained individuals, the majority have not adjusted for MAP and/or heart rate when necessary and have reported lower aPWV in well-trained individuals (Vaitkevicius et al. 1993; McDonnell et al. 2013; Tarumi et al. 2013; Pierce et al. 2013, 2016). However, Shibata et al. (2018) reported no effects of lifelong exercise “dose” on aortic stiffness in middle-aged/older individuals. Unlike aPWV, AP, or more commonly reported augmentation index (AIx), is always adjusted for heart rate and is found to be lower in well-trained individuals (Vaitkevicius et al. 1993; McDonnell et al. 2013; Tarumi et al. 2013; Pierce et al. 2013, 2016; Shibata et al. 2018). Furthermore, not all studies include height or MAP (Stoner et al. 2014) as covariates when comparing AP or AIx between groups. Thus, to the best of our knowledge, no study has reported on the effects of habitual endurance exercise on aPWV and AP, with appropriate statistical adjustments for potential confounders.
As well as habitual exercise, sympathetic vasomotor outflow to the skeletal muscle (i.e. muscle sympathetic nerve activity [MSNA]) could also influence aortic haemodynamics. MSNA is well known to play a pivotal role in the dynamic beat-by-beat control of peripheral vascular tone, and therefore peripheral blood pressure (Charkoudian and Wallin 2014; Shoemaker et al. 2015; Dampney 2017). However, less is known about whether MSNA also influences aortic haemodynamics. Previously, basal MSNA burst frequency and incidence have been shown to positively correlate with AP in normotensive young men (Casey et al. 2011; Smith et al. 2015), postmenopausal women with elevated blood pressure (i.e. [pre]hypertension) (Hart et al. 2013) and normotensive heart failure patients with reduced ejection fraction (Millar et al. 2019). In contrast, MSNA and AP were not correlated in a mixed-sex sample of healthy normotensive middle-aged individuals (Millar et al. 2019). Casey et al. (2011) suggested that MSNA likely influences AP via alterations in peripheral vascular tone, and subsequent increases in the magnitude of backward travelling pressure waves, as well as increases in aortic stiffness. Importantly, in young normotensive men, there is often no mid-systolic rise in aortic pressure (McEniery et al. 2005) and therefore AP is either zero or negative (Fig. 1A), unlike older normotensive men (Fig. 1B). Thus, in young men AP does not contribute to aSBP and therefore is not physiologically meaningful in the context of effecting increases in aSBP. Furthermore, inclusion of negative AP values can exaggerate relationships between variables (e.g. between AP and age), whereby this relationship does not exist following the removal of these values (Hughes et al. 2013). In the two aforementioned studies of young men, both negative and positive AP values were included in analyses (Casey et al. 2011; Smith et al. 2015); accordingly, the reported positive correlation between MSNA and AP may be exaggerated.
Herein, we aimed to (1) establish whether appropriate statistical adjustment influences the findings as to the effects of habitual endurance exercise on aortic haemodynamics, and, (2) assess the relationship between MSNA burst frequency and AP in normotensive young and middle-aged men. For aim 1, we hypothesised that adjusted aPWV and AP would not be different between groups due to training-mediated effects on heart rate and/or blood pressure, and that the age-related differences in aortic haemodynamics would be smaller in runners compared to nonrunners. To address aim 2, we merged the endurance-trained and recreationally active groups as MSNA burst frequency is not influenced by habitual exercise in either age group (Wakeham et al. 2019). Furthermore, we performed separate analyses for each age group due to higher MSNA and positive AP values in middle age (Matsukawa et al. 1998; McEniery et al. 2005). We hypothesised that there would be no significant correlation between MSNA and AP in either age group.
Methodology
Study overview
The individuals studied here have been reported on previously to address separate a priori study aims (Wakeham et al. 2019; Lord et al. 2020; Talbot et al. 2020). Forty-six healthy (free of chronic disease), non-smoking, normotensive and non-obese males were recruited into groups of endurance-trained runners and recreationally active nonrunners. Young runners had been training for 8 ± 5 years and were currently completing 65 ± 14 miles per week. The middle-aged runners had been training for 29 ± 15 years and were currently completing 35 ± 10 miles of per week. All endurance-trained runners were ranked within the top 30% of their age-category in the UK for 5 km in the year in which they were studied, with season best times of 15:22 ± 00:37 min:s and 19:31 ± 00:57 min:s for young and middle-aged runners, respectively. Nonrunners were recruited based upon self-report of completing at least 150 min of moderate to vigorous physical activity per week in accordance with the UK Physical Activity Guidelines for adults aged 19–64 years of age from the United Kingdom Chief Medical Officers (2019), but were not undertaking structured exercise training. All middle-aged men presented with a normal electrocardiogram at rest and during a maximal exercise test (via an incremental ramp protocol) on a cycle ergometer (Lode Corival, Groningen, Netherlands). Maximal exercise tests were used to determine cardiorespiratory fitness (\(\dot{V}{\text{O}}_{2}\) Peak), via automated indirect calorimetry (Oxycon Pro, Jaeger, Hoechberg, Germany); the Oxycon Pro has been validated across a range of exercise intensities (Rietjens et al. 2001). This study conformed to the Declaration of Helsinki, other than registration in a database, and all procedures were approved by the Cardiff School of Sport and Health Sciences Research Ethics Committee (16/7/02R). Prior to testing, all participants provided both written and verbal informed consent.
Study design
All visits were completed in a temperature-controlled laboratory (~ 22 °C). Participants attended the laboratory on two occasions having abstained from caffeine, alcohol, and strenuous exercise for twenty-four hours and fasted for six hours. The first visit involved the assessment of aortic haemodynamics, followed by the measurement of \(\dot{V}{\text{O}}_{2}\) Peak. On a separate day, with a minimum of 1 week between testing days, participants underwent recordings of MSNA at rest, as outlined previously (Wakeham et al. 2019).
Experimental measurements and data analyses
Aortic haemodynamics
The assessment of aortic haemodynamics was conducted as detailed previously (Talbot et al. 2020). Briefly, participants rested supine for 15 min prior to the assessment of brachial arterial blood pressure (Welch Allyn, UK) which was followed by the collection of radial arterial waveforms by applanation tonometry, using a high-fidelity micromanometer tipped probe (Millar Instruments), to estimate ascending aortic blood pressure (SphygmoCor, AtCor Medical, Australia). We determined the following parameters from the ascending aortic waveform, which was estimated using a generalised inverse transfer function (Pauca et al. 2001): P1 (first systolic shoulder of the aortic waveform; referred to as non-augmented aSBP), P2 (second systolic shoulder of the aortic waveform), AP (P2 – P1, mmHg), AIx ([AP/aortic pulse pressure] × 100, %), aSBP, aortic diastolic blood pressure (aDBP), aortic pulse pressure (aPP) and aortic mean arterial pressure (aMAP; Fig. 1). AP and AIx were also extracted following adjustment for a heart rate of 75 bpm (AP@75 and AIx@75, respectively) within the SphgymoCor software, which is calculated based upon the regression equations from Wilkinson and colleagues (2000; 2002), as detailed previously (Stoner et al. 2014). We also quantified pulse pressure amplification (brachial PP-aPP). All data were collected in triplicate in accordance with the quality criteria within the SphygmoCor software by one trained observer (TGD), with an average reported herein. AP is the primary index of aortic systolic pressure augmentation in this study as AP increases linearly with age (McEniery et al. 2005) and is not influenced mathematically by the concurrent increase in aPP, unlike AIx (Namasivayam et al. 2010). We consider AP as an index of aortic systolic pressure augmentation only, not aortic reservoir pressure nor the magnitude of backward travelling pressure waves per se (Hughes et al. 2013).
Effects of aortic stiffness and augmentation pressure on the ascending aortic blood pressure waveform. A, B These panels represent the effects of differences in aortic stiffness and augmentation pressure on aortic blood pressure and systolic pressure augmentation. Augmentation pressure (AP) and index (AIx) quantify systolic pressure augmentation; the equations to calculate AP and AIx are shown in the box within (B). Pressure 1 (P1) is taken as aortic systolic blood pressure (aSBP) in (A), whereas pressure 2 (P2) is recorded as aSBP in (B), due to positive systolic pressure augmentation in this instance. Aortic diastolic pressure is determined from the nadir of the waveform immediately prior to ventricular ejection which leads to the upstroke of the pressure waveform. Aortic valve closure (AVC) is noted at the incisura, which represents the end of ventricular ejection. AIx augmentation index, AP augmentation pressure, aPP aortic pulse pressure, AVC aortic valve closure, P1 pressure 1, P2 pressure 2
Aortic (aPWV) and brachial pulse wave velocity (bPWV) were then determined in accordance with current guidelines (Townsend et al. 2015), by dividing the transit time between the foot of the carotid and femoral, or radial, arterial waveforms, respectively, by the path length. To enable comparison of data with the published reference values (Reference Values for Arterial Stiffness, 2010), raw aPWV was converted using the following equation: converted aPWV = (raw carotid-femoral distance × 1.12) ÷ time (Van Bortel et al. 2012; Huybrechts et al. 2011). Both raw and converted aPWV are reported in line with recent suggestions (Van Bortel et al. 2020). We also quantified the arterial stiffness gradient as the ratio of raw bPWV and converted aPWV (Niiranen et al. 2017).
Raw AP and aPWV are influenced by heart rate, (a) MAP, age and height (for AP only) (Stoner et al. 2014; Tan et al. 2016; Van Bortel et al. 2020). In the presence of significant differences in these covariates, AP and aPWV are also presented in adjusted form (via analysis of covariance [ANCOVA]), to determine the effects of habitual exercise and age on intrinsic aortic stiffness (aPWV) and associated haemodynamics (AP). If converted aPWV differs between groups there would be a functionally relevant difference in operating aortic stiffness, as this measure reflects windkessel function which will contribute to haemodynamic regulation. However, if adjusted aPWV is not different between groups this would suggest that there is no difference in intrinsic aortic stiffness (i.e. aPWV independent of confounders) and that the difference in operating aortic stiffness is mediated by the factors adjusted for. This is similar for AP; whereby, if measured AP differs between groups this additional haemodynamic load if faced by the left ventricle; however, if this difference does not persist for adjusted AP it highlights that this difference is mediated by the factors adjusted for. As age is an independent variable in this study, age was not included as a covariate. Raw bPWV was also statistically adjusted (via separate ANCOVAs) for MAP and heart rate. The adjustment for aPWV and bPWV for aMAP and MAP, respectively, was due to the significant difference between aMAP and MAP in young but not middle-aged men (Young: mean difference [95% confidence interval], − 4 [− 6 to − 2] mmHg, P < 0.001; Middle-aged: − 1 [− 2 to 1] mmHg, P = 0.422; via independent-samples t test).
Muscle sympathetic nerve activity
Recordings of multi-unit MSNA were taken from the peroneal nerve using microneurography, as previously described (Wakeham et al. 2019). In brief, a tungsten microelectrode was inserted into the peroneal nerve, by one trained microneurographer (JPM), with a reference electrode inserted 2–3 cm away to obtain the raw nerve signal which was amplified, filtered, rectified and integrated (time constant 0.1 s; Nerve Traffic Analyser, Model 663 C, Iowa). MSNA was sampled at 1 kHz onto a personal computer using a commercially available data acquisition system (Chart Version 8, LabChart Pro, ADInstruments, UK) and saved for offline analysis. MSNA was analysed as previously described (Wakeham et al. 2019) and is presented as burst frequency (bursts min−1) and burst incidence (bursts 100 hb−1) for the assessment of between-group differences. Notably, MSNA burst frequency is the primary MSNA variable of interest for the correlational analyses, as it reflects the temporal pattern of sympathetic outflow to the vasculature which determines neurotransmitter release; whereas, MSNA burst incidence reflects the arterial baroreflex control of MSNA (Charkoudian and Wallin 2014).
Statistical analyses
To address the first aim of this study, after confirming compliance with basic parametric assumptions, analysis of variance (ANOVA) was used to compare participant characteristics. Subsequently, a general linear model was used to compare aortic haemodynamics between groups; that is to determine the main effects of runner and age, and the runner*age interaction. In the presence of a significant runner*age interaction, SIDAK post-hoc multiple comparisons were assessed. In the presence of significant main effects of runner or age for Converted aPWV or AP, as well as for the potential covariates (heart rate and aMAP [or height, for AP only]), then ANCOVA analyses were performed. The statistical model used for the ANCOVA was dependent on the presence of a significant runner*age interaction. If there was no significant runner*age interaction, then a general linear model was used with the inclusion of the significant covariates; however, if there was a significant interaction, separate one-way “posthoc” ANCOVA analyses were conducted for each of the four primary between-group comparisons (i.e. effects of runner in young [1] and middle-aged men [2], as well as the effects of age in nonrunners [3] and runners [4]). Adjusted group standard deviations (SD) were calculated from the sum of the square root of the sample size and the standard error of the estimated marginal means from the adjusted statistical models (Atkinson and Batterham 2013). Mean difference [95% confidence intervals] and Hedges’ g, an estimate of effect size ([−]0.2 small effect, [−]0.5 medium effect, [−]0.8 large effect) (Cohen 1992), are also reported for primary outcomes, namely adjusted aPWV, adjusted AP, and aSBP. To address the second aim of this study, two-tailed Pearson product-moment correlation coefficients were generated to assess the relationship between MSNA and AP for the merged samples of young (n = 23) and middle-aged (n = 23) men. Data are presented as mean ± standard deviation or as mean difference [95% confidence intervals] and α was set a priori at < 0.05. All statistical analyses were conducted using Statistics Package for Social Sciences (SPSS) for Windows (version 26, Chicago, IL).
Results
Participant characteristics
In both young and middle-aged men, body mass and BMI were significantly lower in runners compared to nonrunners, whereas \(\dot{V}{\text{O}}_{2}\) Peak was higher in runners (Table 1). \(\dot{V}{\text{O}}_{2}\) Peak was lower in middle-aged compared to young runners, with no difference between middle-aged and young nonrunners.
Study aim 1: effects of habitual exercise and age on aortic haemodynamics
Blood pressure
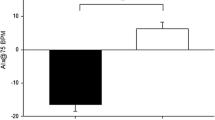
Heart rate, non-augmented aSBP, aMAP, DBP and MAP were lower in runners compared to nonrunners (main effect of runner); whereas, non-augmented aSBP, aSBP, aDBP, aPP, aMAP, DBP, MAP and PPA were higher with age (main effect of age; Table 2). There was a significant runner*age interaction for aSBP (Fig. 2A), whereby aSBP was lower in young runners compared to nonrunners (− 8 [− 13 to − 3] mmHg, Hedges’ g = 1.1) with no difference between middle-aged runners and nonrunners (3 [− 3 to 8] mmHg, Hedges’ g = − 0.3). Thus, there was a larger mean difference in aSBP with age in runners (18 [13 to 23] mmHg, Hedges’ g = 2.6) compared to nonrunners (8 [2–14] mmHg, Hedges’ g = 1.1).
Effects of habitual exercise and age on aortic haemodynamics. A There was a significant runner*age interaction for aSBP. Specifically, aSBP was lower in young runners compared to nonrunners, with no difference between middle-aged groups; thus, the age-related difference was greater in runners compared to nonrunners (see text). B Age, but not habitual endurance exercise, increased adjusted aPWV; whereby aortic stiffness was higher with age in both runners and nonrunners. C There was no effect of habitual exercise on adjusted AP; however, age was associated with higher systolic pressure augmentation. As detailed within text, the magnitude of difference with age was greater in runners compared to nonrunners. NB: All data are presented with standard deviation error bars. The main effects and interaction, with SIDAK post hoc tests of multiple comparisons are presented from a general linear model for aSBP (A). Covariates, noted in [parentheses], were added to the general linear model to determine between-group differences in Adjusted aPWV (B) and AP (C). Separate ANCOVA analyses were conducted to determine between-group differences in Adjusted AP due to the significant runner*age interaction (C). AP augmentation pressure, aPWV aortic pulse wave velocity, aMAP aortic mean arterial pressure, aSBP aortic systolic blood pressure, HR heart rate
Arterial stiffness
Converted aPWV (i.e. unadjusted) was lower in runners compared to nonrunners and higher in middle-aged compared to young men (Table 2). However, adjusted aPWV was not different between young (− 0.5 [− 1.2 to 0.3] m s−1, Hedges’ g = -0.5) or middle-aged (− 0.5 [− 1.3 to 0.3] m s−1, Hedges’ g = -0.6) runners and nonrunners, but was higher in middle-aged men (nonrunners: 1.7 [0.9–2.5] m s−1, Hedges’ g = 1.6; runners: 1.7 [0.9–2.4] m s−1, Hedges’ g = 1.9); there was no significant runner*age interaction (Fig. 2B). Results were similar for raw and adjusted bPWV; whereby, bPWV was lower in runners compared to nonrunners and higher with age, with no runner*age interaction. The arterial stiffness gradient (bPWV-aPWV) was higher with age only (Table 2).
Systolic pressure augmentation
There was a significant runner*age interaction for raw AP. Specifically, raw AP was higher in middle-aged runners compared to nonrunners (P < 0.001), with no significant difference between young groups (P = 0.360); thus, the age-related difference was greater in runners (13 [11–15] mmHg, P < 0.001) compared to nonrunners (8 [5–10] mmHg, P < 0.001). When adjusted via ANCOVA, AP (Fig. 2C) was not different between runners and nonrunners in either the young (− 2 [− 4 to 1] mmHg, Hedges’ g = − 0.6) or middle-aged groups (2 [− 1 to 4] mmHg; Hedges’ g = 0.6), but was higher with age in nonrunners (8 [5–11] mmHg, Hedges’ g = 2.6) and runners (11 [8–13] mmHg, Hedges’ g = 3.6), with a slightly larger effect in runners, similar to the finding for raw AP. Notably, these results differ as to the effects of habitual exercise when using either AP@75 (or AIx@75); whereby, AP@75 is lower in young runners compared to nonrunners (P < 0.001) but not different between middle-aged runners and nonrunners (P = 0.420).
Study aim 2: relationships between MSNA and AP
When the runners and nonrunners were merged within each age group to address the second study aim, middle-aged men were older (55 ± 4 vs 23 ± 3 years, P < 0.001) and shorter (175.1 ± 6.5 vs 179.1 ± 5.4 cm, P = 0.025) than young men. Body mass (72.6 ± 11.4 vs 72.8 ± 12.9 kg, P = 0.946), BMI (24 ± 3 vs 23 ± 4 kg m2, P = 0.433) and \(\dot{V}{\text{O}}_{2}\) Peak (43.8 ± 10.9 vs 50.1 ± 14.6 mL kg−1 min−1, P = 0.067) were not different between middle-aged and young men. MSNA burst frequency (30 ± 10 vs 17 ± 8 bursts min−1, P < 0.001) and incidence (62 ± 21 vs 32 ± 18 bursts 100hb−1, P < 0.001) were higher in middle-aged men.
As 4 young men had AP ≥ 0 and 1 middle-aged man had an AP of 0, separate analyses for positive and negative AP values was not possible in each age group. Correlation analyses were, therefore, conducted in 19 young men and 22 middle-aged men, with only negative and positive AP values, respectively. We observed no significant relationship between MSNA burst frequency and AP in either young or middle-aged men (Fig. 3). In addition, there was no significant correlation between MSNA and AIx in either young (r = − 0.03, P = 0.893) or middle-aged (r = 0.12, P = 0.574) men.
The relationship between muscle sympathetic nerve activity and aortic augmentation pressure. There were no significant correlations between muscle sympathetic nerve activity (MSNA) burst frequency and augmentation pressure in young (A; n = 19) or middle-aged (B; n = 21) normotensive men. 5 participants were excluded from these analyses (4 young [2 runners] and 1 middle-aged nonrunner) due to AP values ≥ 0 in the young group or ≤ 0 in the middle-aged group. NB: Dotted lines represent the 95% confidence intervals. Open symbols represent the runners within each age group
Discussion
The principal findings from this study are as follows. First, the statistical adjustment of aPWV and AP for aMAP and heart rate changed the conclusions as to the effects of habitual endurance; adjusted aPWV and AP were not different between trained and untrained men, which contrasts the lower unadjusted values of Converted aPWV and AP. Second, there was an age-dependent effect of habitual exercise on aSBP, whereby aSBP was lower in young runners compared to nonrunners only. Accordingly, the magnitude of difference in aSBP was greater with age in runners compared to nonrunners, with a similar age-related difference for aPWV and AP. Third, we report no significant relationship between MSNA burst frequency, in other words sympathetic vasomotor outflow, and AP in young or middle-aged men, with negative and positive AP, respectively. These data suggest that habitual exercise is associated with a different aortic haemodynamic profile in young compared to middle-aged men and habitual endurance exercise does not influence aortic stiffness or augmentation pressure when adjusted for the physiological effects of heart rate and blood pressure. Furthermore, the data presented here show that aortic systolic pressure augmentation does not correlate with the level of sympathetic vasoconstrictor drive to skeletal muscle in groups of active young or middle-aged normotensive men.
Effects of habitual exercise on aortic haemodynamics in young and middle-aged men
In the present study, HR and aMAP adjusted aPWV was not influenced by habitual exercise in either young or middle-aged men. In the studies conducted to date aPWV has not been adjusted for HR and aMAP (Vaitkevicius et al. 1993; Tanaka et al. 1998; McDonnell et al. 2013; Pierce et al. 2013, 2016; Tarumi et al. 2013, 2021). Notably, the adjustment of aPWV markedly changed our study findings. Converted aPWV (i.e. unadjusted) was lower with habitual exercise in both young and middle-aged men, as reported in most (Vaitkevicius et al. 1993; Tanaka et al. 1998; McDonnell et al. 2013; Pierce et al. 2013, 2016; Tarumi et al. 2013, 2021; Otsuki et al. 2007a, b), but not all (Bjarnegard et al. 2018; McDonnell et al. 2013; Shibata et al. 2018) previous studies. Our findings suggest that habitual exercise may not directly influence intrinsic aortic stiffness (i.e. aPWV independent of confounders) per se, rather that habitual exercise influences operating aortic stiffness (i.e. measured aPWV) due to training-related effects on heart rate and arterial pressure. Thus, future studies should also determine adjusted aPWV when appropriate, dependent on differences in heart rate and/or arterial pressure, to avoid reporting erroneous conclusions (Van Bortel et al. 2020) regarding the effects of habitual exercise on intrinsic aortic stiffness. These data suggest that habitual exercise does not change intrinsic aortic stiffness in healthy normotensive men.
Alongside aortic stiffness, aortic systolic pressure augmentation is an important determinant of aortic blood pressure, which independently increases cardiovascular risk (Wang et al. 2010). When adjusted for heart rate and aMAP, there were no significant effects of habitual exercise on AP (or AIx) in young or middle-aged men. Again, these findings differ when these variables are unadjusted, as AP was not different in young men, but AP was higher in middle-aged runners compared to nonrunners. AP@75 was, however, lower in young runners compared to nonrunners, whereas the normalisation to a heart rate of 75 bpm removed the difference between middle-aged groups. Thus, the adjustment of AP for heart rate and aMAP highlights the importance of including aMAP as a covariate when comparing AP between the trained and untrained men here, as adjusted AP was not effected by habitual exercise in either age group. The use of ANCOVA for the adjustment of AP or AIx for heart rate, as opposed to the automated adjusted to 75 bpm within the SphygmoCor, is beneficial as this statistical method adjusts the dependent variable according to the group means and not based upon an arbitrary calculation from previous data, as highlighted previously (Stoner et al. 2014). Furthermore, the adjustment to 75 bpm is limited to heart rates between 40 and 110; thus, it is not possible to generate these data in endurance athletes who present with heart rates below 40 bpm, as was the case for four runners included here. Previous studies have reported that systolic pressure augmentation (AP, AIx or AIx@75) is either lower (Edwards and Lang 2005; Denham et al. 2016; McDonnell et al. 2013; Bjarnegard et al. 2018) or not different (Knez et al. 2008; McDonnell et al. 2013; Shibata et al. 2018) with habitual exercise. The mechanism(s) mediating the higher raw AP in middle-aged runners compared to nonrunners are unclear; future studies are required to determine the influence of habitual exercise across the lifespan on forward, backward and reservoir pressures. The disparity in findings is likely due to the differences in the measure (AP vs AIx), or equipment used, whether, or not, heart rate and aMAP were adjusted for, as well as the magnitude of differences in cardiorespiratory fitness and/or participant age. Together, the data presented here suggest that the habitual endurance exercise related difference in aortic systolic pressure augmentation is mediated by heart rate and aMAP.
The effects of habitual exercise on aSBP were age-dependent; whereby, aSBP was only lower in young runners compared to nonrunners, despite no significant differences in adjusted aPWV or AP in either age group. This age-dependent effect is likely due to the greater magnitude of difference in non-augmented aSBP (i.e. P1) in young runners compared to nonrunners (-6 mmHg); the mean difference was smaller (− 2 mmHg) between middle-aged groups. As P1 is determined by the interaction between the left ventricular ejection wave and ascending aortic stiffness, the greater ascending aortic compliance in young endurance-trained men (Tarumi et al. 2021) likely contributes to this difference in non-augmented aSBP. The effects of habitual exercise on ascending aortic stiffness in middle/older age are currently unclear. It is important to note that our data do contradict a previous study which reported the inverse, with effects of habitual exercise on aSBP in middle-aged but not young individuals (McDonnell et al. 2013). However, this discrepancy is likely related to the study of only normotensive men in both age groups here, unlike the previous study. Therefore, in terms of cardiovascular risk as determined by aSBP (Stamatelopoulos et al. 2006; Vlachopoulos et al. 2010; Lamarche et al. 2020), the benefit of habitual endurance exercise is apparent in young but not middle-aged normotensive men.
Effect of age in runners and nonrunners
With advancing age aortic stiffness, systolic pressure augmentation, and aortic blood pressure increase (McEniery et al. 2005). Many studies have suggested that lifelong habitual exercise can slow age-related aortic stiffening (Vaitkevicius et al. 1993; Tanaka et al. 1998; Gates et al. 2003; Pierce et al. 2013, 2016; McDonnell et al. 2013), which could also influence AP. However, some of these previous studies compared middle/older aged endurance-trained individuals with young sedentary, but not young endurance-trained, individuals (Vaitkevicius et al. 1993; Pierce et al. 2013, 2016), which could underestimate the age-related difference with the middle/older aged endurance-trained groups. Indeed, we report that the age-related differences in aPWV are similar for chronically endurance-trained runners and recreationally-active nonrunners. Importantly, the middle-aged runners here had been training for ~ 30 years, which is similar to the age difference between our young and middle-aged groups (~ 32 years). This finding opposes the view that habitual endurance exercise has “anti-ageing” effects on aortic stiffness, as reviewed recently (Nowak et al. 2018; Rossman et al. 2018; Seals et al. 2018; Tanaka 2019; Seals et al. 2019; Parry-Williams and Sharma 2020). The age-related difference in AP was greater (larger effect size) in runners compared to nonrunners. This also contradicts a previous report of no effect of habitual exercise on the age-related difference in AP has been reported previously (McDonnell et al. 2013); however, as mentioned previously, the older groups studied by McDonnell and colleagues were prehypertensive and hypertensive, unlike the present study, and the active group were likely not as homogenous as the sample studied here. Thus, more studies are warranted to provide further insight into whether habitual endurance exercise influences “aortic ageing”, utilising a similar four-group study design as utilised here, with appropriate adjustments of aPWV and AP.
In the present study, the age-related difference in aSBP was greater in runners compared to nonrunners. Despite this, the middle-aged runners did not present with higher aSBP or adjusted AP when compared to their recreationally active age-matched counterparts (Table 2). This is of importance for their cardiovascular risk profile, as absolute aSBP is predictive of cardiovascular morbidity and mortality (Stamatelopoulos et al. 2006; Vlachopoulos et al. 2010; Lamarche et al. 2020).
Relationship between MSNA and AP
In the current study, we found no relationship between MSNA burst frequency and AP, in young or middle-aged normotensive men. Specifically, we observed this in a sample of young men where AP did not contribute to aSBP in 91% of individuals, but did contribute to aSBP in 96% of middle-aged men, due to the return of the backward pressure wave occurring in diastole and systole in young and middle-aged men, respectively. MSNA and AP (or AIx) have been shown to positively correlate in young men (Casey et al. 2011; Smith et al. 2015); however, 44% (Casey et al. 2011) and 53% (Smith et al. 2015) of the samples studied previously had positive AP values. Whether these previously reported correlations between MSNA and AP in young men would remain when assessed for positive and negative AP values separately, is unclear. The previous studies in middle/older age report disparate findings. Millar et al. (2019) reported no correlation between MSNA and AP in healthy normotensive individuals (75% male), where AP contributed to aSBP in 88% of participants studied. The discordance in findings between this study and those conducted previously likely reflects differences in the distribution of AP values and/or levels of aortic pressure. Indeed, higher arterial pressure is associated with higher AP (Mitchell 2014). Positive relationships between MSNA and AP may only exist in individuals with elevated arterial pressure and AP, as shown previously in prehypertensive and hypertensive postmenopausal women (Hart et al. 2013) and heart failure patients with reduced ejection fraction (Millar et al. 2019). Together, in normotensive young or middle-aged men, without and with pressure raising AP values respectively, resting peripheral sympathetic vasomotor outflow does not correlate with AP (or AIx).
Casey et al. (2011) suggested that MSNA likely contributes to AP in young men via alterations in peripheral vascular tone, and subsequent increases in backward travelling pressure waves, as well as increases in aortic stiffness (Casey et al. 2011). However, the authors made this conclusion despite reporting no significant correlation between total peripheral resistance and AP in young men (Casey et al. 2011). Regarding the influence of MSNA on aortic stiffness, and subsequently AP, it has been shown that MSNA significantly correlates with aPWV at rest (Swierblewska et al. 2010; Holwerda et al. 2019) and there are pressure-independent increases in aPWV during acute sympathoexcitation (Nardone et al. 2018; Holwerda et al. 2019). Notably, we observe no significant correlation between MSNA and aPWV in either age group studied here (young: r = 0.162, P = 0.461; middle-aged: r = − 0.367, P = 0.085), which may contribute to the lack of relationships between MSNA and AP in this study. Thus, a positive relationship between MSNA and aortic stiffness may underlie the previously reported positive correlations between MSNA and AP (Casey et al. 2011; Smith et al. 2015; Hart et al. 2013; Millar et al. 2019); an effect likely due to the pressure-raising effect higher aortic stiffness has upon aortic reservoir pressure, and subsequently AP (Davies et al. 2010). This may especially be the case in the presence of high blood pressure and/or cardiovascular disease where AP raises aSBP (Mitchell 2014).
Methodological considerations
There are some limitations which warrant discussion. First, as we only studied Caucasian men here it is not possible to determine if there are similar or divergent effects of habitual exercise and/or ageing on aortic haemodynamics between the sexes or different racial groups. The effects of habitual exercise on central haemodynamics have been studied in women previous, with reports of no differences between trained and untrained women in either young (Bjarnegard et al. 2018) or middle/older age (Tanaka et al. 1998). Further, there are no effects of sex on aortic pressure in either African American or Caucasian individuals, despite lower aPWV in women (Yan et al. 2014). Future studies are required to understand the interaction between the effects of habitual exercise, age, sex, and other socio-cultural factors on aortic haemodynamics. Second, as a cross-sectional study design was utilised, it is not possible to truly determine the effects of habitual exercise on the age-related changes in aortic haemodynamics. Future studies should complete longitudinal follow-up studies in endurance-trained and untrained men and women to discern the effects of age, sex and habitual exercise on aortic haemodynamics. Furthermore, as we did not record objective measures of physical activity here, we cannot determine the effects of different physical activity and/or sedentary time on aortic haemodynamics. This was by design, as we studied healthy but untrained men who were meeting the current physical activity guidelines. Third, we assessed the relationships between resting MSNA and aortic AP which were recorded on separate days. However, the measurement of MSNA is repeatable within individuals over the short, medium and long-term (Fagius and Wallin 1993; Kimmerly et al. 2004; Notay et al. 2016; Grassi et al. 1997; Fonkoue and Carter 2015). Finally, it is not possible to directly record sympathetic outflow to the aorta in humans. Nevertheless, the main aim of the correlational analyses performed here was to determine whether peripheral sympathetic vasomotor outflow (i.e. MSNA) is correlated to AP with consideration of the effect of negative and positive AP values.
Conclusion
We report an age-dependent effect of habitual endurance exercise on aortic haemodynamics in normotensive men; whereby, aSBP is lower in young, but not middle-aged, runners compared to nonrunners, with no difference in adjusted aPWV or AP in either age-group. The difference in findings between the raw and adjusted aPWV and AP data highlight that the appropriate adjustments of aortic stiffness and systolic pressure augmentation are important when studying the effects of habitual exercise between groups of young and middle-aged men to avoid reporting erroneous conclusions (Van Bortel et al. 2020). However, the differences in unadjusted aPWV and AP with habitual exercise reported here are the functionally relevant operating aortic stiffness and augmentation pressure values, which are of clear physiological and potential clinical relevance. Nevertheless, our study highlights that intrinsic aortic stiffness and adjusted systolic augmentation pressure are not influenced by habitual exercise. Thus, the habitual exercise related differences in operating aortic stiffness and systolic augmentation pressure appear to be mediated by differences in other hemodynamic indices, namely heart rate and blood pressure. In addition, we report no significant correlation between peripheral sympathetic vasomotor outflow and systolic pressure augmentation in either young or middle-aged normotensive men. Future studies should consider performing separate correlational analyses with positive and negative AP values, as only positive AP values represent clinically relevant physiological effects on aSBP.
Availability of data and material
Data are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
References
Atkinson G, Batterham AM (2013) The percentage flow-mediated dilation index: a large-sample investigation of its appropriateness, potential for bias and causal nexus in vascular medicine. Vasc Med 18(6):354–365. https://doi.org/10.1177/1358863X13508446
Bjarnegard N, Lanne T, Cinthio M, Ekstrand J, Hedman K, Nylander E, Henriksson J (2018) Vascular characteristics in young women-Effect of extensive endurance training or a sedentary lifestyle. Acta Physiol (oxf) 223(2):e13041. https://doi.org/10.1111/apha.13041
Casey DP, Curry TB, Joyner MJ, Charkoudian N, Hart EC (2011) Relationship between muscle sympathetic nerve activity and aortic wave reflection characteristics in young men and women. Hypertension 57(3):421–427. https://doi.org/10.1161/HYPERTENSIONAHA.110.164517
Charkoudian N, Wallin BG (2014) Sympathetic neural activity to the cardiovascular system: integrator of systemic physiology and interindividual characteristics. Compr Physiol 4(2):825–850. https://doi.org/10.1002/cphy.c130038
Cohen J (1992) A power primer. Psychol Bull 112(1):155–159. https://doi.org/10.1037//0033-2909.112.1.155
Dampney RAL (2017) Resetting of the Baroreflex Control of Sympathetic Vasomotor Activity during Natural Behaviors: Description and Conceptual Model of Central Mechanisms. Front Neurosci 11:461. https://doi.org/10.3389/fnins.2017.00461
Davies JE, Baksi J, Francis DP, Hadjiloizou N, Whinnett ZI, Manisty CH, Aguado-Sierra J, Foale RA, Malik IS, Tyberg JV, Parker KH, Mayet J, Hughes AD (2010) The arterial reservoir pressure increases with aging and is the major determinant of the aortic augmentation index. Am J Physiol Heart Circ Physiol 298(2):H580-586. https://doi.org/10.1152/ajpheart.00875.2009
Denham J, Brown NJ, Tomaszewski M, Williams B, O’Brien BJ, Charchar FJ (2016) Aortic augmentation index in endurance athletes: a role for cardiorespiratory fitness. Eur J Appl Physiol 116(8):1537–1544. https://doi.org/10.1007/s00421-016-3407-x
Edwards DG, Lang JT (2005) Augmentation index and systolic load are lower in competitive endurance athletes. Am J Hypertens 18(5 Pt 1):679–683. https://doi.org/10.1016/j.amjhyper.2004.11.028
Fagius J, Wallin BG (1993) Long-term variability and reproducibility of resting human muscle nerve sympathetic activity at rest, as reassessed after a decade. Clin Auton Res 3(3):201–205. https://doi.org/10.1007/bf01826234
Fonkoue IT, Carter JR (2015) Sympathetic neural reactivity to mental stress in humans: test-retest reproducibility. Am J Physiol Regul Integr Comp Physiol 309(11):R1380-1386. https://doi.org/10.1152/ajpregu.00344.2015
Gates PE, Tanaka H, Graves J, Seals DR (2003) Left ventricular structure and diastolic function with human ageing. Relation to habitual exercise and arterial stiffness. Eur Heart J 24(24):2213–2220. https://doi.org/10.1016/j.ehj.2003.09.026
Grassi G, Bolla G, Seravalle G, Turri C, Lanfranchi A, Mancia G (1997) Comparison between reproducibility and sensitivity of muscle sympathetic nerve traffic and plasma noradrenaline in man. Clin Sci (lond) 92(3):285–289. https://doi.org/10.1042/cs0920285
Hart EC, Charkoudian N, Joyner MJ, Barnes JN, Curry TB, Casey DP (2013) Relationship between sympathetic nerve activity and aortic wave reflection characteristics in postmenopausal women. Menopause 20(9):967–972. https://doi.org/10.1097/GME.0b013e3182843b59
Holwerda SW, Luehrs RE, DuBose L, Collins MT, Wooldridge NA, Stroud AK, Fadel PJ, Abboud FM, Pierce GL (2019) Elevated muscle sympathetic nerve activity contributes to central artery stiffness in young and middle-age/older adults. Hypertension 73(5):1025–1035. https://doi.org/10.1161/HYPERTENSIONAHA.118.12462
Hughes AD, Park C, Davies J, Francis D, Mc GTSA, Mayet J, Parker KH (2013) Limitations of augmentation index in the assessment of wave reflection in normotensive healthy individuals. PLoS ONE 8(3):e59371. https://doi.org/10.1371/journal.pone.0059371
Huybrechts SA, Devos DG, Vermeersch SJ, Mahieu D, Achten E, de Backer TL, Segers P, van Bortel LM (2011) Carotid to femoral pulse wave velocity: a comparison of real travelled aortic path lengths determined by MRI and superficial measurements. J Hypertens 29(8):1577–1582. https://doi.org/10.1097/HJH.0b013e3283487841
Katona PG, McLean M, Dighton DH, Guz A (1982) Sympathetic and parasympathetic cardiac control in athletes and nonathletes at rest. J Appl Physiol Respir Environ Exerc Physiol 52(6):1652–1657. https://doi.org/10.1152/jappl.1982.52.6.1652
Kimmerly DS, O’Leary DD, Shoemaker JK (2004) Test-retest repeatability of muscle sympathetic nerve activity: influence of data analysis and head-up tilt. Auton Neurosci 114(1–2):61–71. https://doi.org/10.1016/j.autneu.2004.06.005
Knez WL, Sharman JE, Jenkins DG, Coombes JS (2008) Central hemodynamics in ultra-endurance athletes. J Sci Med Sport 11(4):390–395. https://doi.org/10.1016/j.jsams.2006.11.005
Lamarche F, Agharazii M, Madore F, Goupil R (2020) Prediction of cardiovascular events by type I central systolic blood pressure: a prospective study. Hypertension. https://doi.org/10.1161/HYPERTENSIONAHA.120.16163
Lord RN, Wakeham DJ, Pugh CJA, Simpson LL, Talbot JS, Lodge FM, Curry BA, Dawkins TG, Shave RE, Moore JP (2020) The influence of barosensory vessel mechanics on the vascular sympathetic baroreflex: insights into aging and blood pressure homeostasis. Am J Physiol Heart Circ Physiol 319(2):H370–H376. https://doi.org/10.1152/ajpheart.00265.2020
Matsukawa T, Sugiyama Y, Watanabe T, Kobayashi F, Mano T (1998) Gender difference in age-related changes in muscle sympathetic nerve activity in healthy subjects. Am J Physiol 275(5 Pt 2):R1600-1604
McDonnell BJ, Maki-Petaja KM, Munnery M, Yasmin WIB, Cockcroft JR, McEniery CM (2013) Habitual exercise and blood pressure: age dependency and underlying mechanisms. Am J Hypertens 26(3):334–341. https://doi.org/10.1093/ajh/hps055
McEniery CM, Yasmin HIR, Qasem A, Wilkinson IB, Cockcroft JR, Investigators A (2005) Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity: the Anglo-Cardiff Collaborative Trial (ACCT). J Am Coll Cardiol 46(9):1753–1760. https://doi.org/10.1016/j.jacc.2005.07.037
Millar PJ, Notarius CF, Haruki N, Floras JS (2019) Heart failure-specific relationship between muscle sympathetic nerve activity and aortic wave reflection. J Card Fail 25(5):404–408. https://doi.org/10.1016/j.cardfail.2019.03.005
Mitchell GF (2014) Arterial stiffness and hypertension: chicken or egg? Hypertension 64(2):210–214. https://doi.org/10.1161/HYPERTENSIONAHA.114.03449
Mynard JP, Kondiboyina A, Kowalski R, Cheung MMH, Smolich JJ (2020) Measurement, analysis and interpretation of pressure/flow waves in blood vessels. Front Physiol 11:1085. https://doi.org/10.3389/fphys.2020.01085
Namasivayam M, Adji A, O’Rourke MF (2010) Aortic augmentation index and aging: mathematical resolution of a physiological dilemma? Hypertension 56(1):e9-10. https://doi.org/10.1161/HYPERTENSIONAHA.110.153742
Nardone M, Incognito AV, Millar PJ (2018) Evidence for pressure-independent sympathetic modulation of central pulse wave velocity. J Am Heart Assoc. https://doi.org/10.1161/JAHA.117.007971
Niiranen TJ, Kalesan B, Larson MG, Hamburg NM, Benjamin EJ, Mitchell GF, Vasan RS (2017) Aortic-Brachial Arterial Stiffness Gradient and Cardiovascular Risk in the Community: The Framingham Heart Study. Hypertension 69(6):1022–1028. https://doi.org/10.1161/HYPERTENSIONAHA.116.08917
Notay K, Seed JD, Incognito AV, Doherty CJ, Nardone M, Burns MJ, Millar PJ (2016) Validity and reliability of measuring resting muscle sympathetic nerve activity using short sampling durations in healthy humans. J Appl Physiol (1985) 121(5):1065–1073. https://doi.org/10.1152/japplphysiol.00736.2016
Nowak KL, Rossman MJ, Chonchol M, Seals DR (2018) Strategies for achieving healthy vascular aging. Hypertension 71(3):389–402. https://doi.org/10.1161/HYPERTENSIONAHA.117.10439
Officers' UCM (2019) Physical Activity Guidelines. https://www.gov.uk/government/publications/physical-activity-guidelines-uk-chief-medical-officers-report. Accessed 07 May 2020
O’Rourke MF, Mancia G (1999) Arterial stiffness. J Hypertens 17(1):1–4. https://doi.org/10.1097/00004872-199917010-00001
Otsuki T, Maeda S, Iemitsu M, Saito Y, Tanimura Y, Ajisaka R, Miyauchi T (2007a) Relationship between arterial stiffness and athletic training programs in young adult men. Am J Hypertens 20(9):967–973. https://doi.org/10.1016/j.amjhyper.2007.05.001
Otsuki T, Maeda S, Iemitsu M, Saito Y, Tanimura Y, Ajisaka R, Miyauchi T (2007b) Vascular endothelium-derived factors and arterial stiffness in strength- and endurance-trained men. Am J Physiol Heart Circ Physiol 292(2):H786-791. https://doi.org/10.1152/ajpheart.00678.2006
Parry-Williams G, Sharma S (2020) The effects of endurance exercise on the heart: panacea or poison? Nat Rev Cardiol. https://doi.org/10.1038/s41569-020-0354-3
Pauca AL, O’Rourke MF, Kon ND (2001) Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension 38(4):932–937
Pierce GL, Casey DP, Fiedorowicz JG, Seals DR, Curry TB, Barnes JN, Wilson DR, Stauss HM (2013) Aortic pulse wave velocity and reflecting distance estimation from peripheral waveforms in humans: detection of age- and exercise training-related differences. Am J Physiol Heart Circ Physiol 305(1):H135-142. https://doi.org/10.1152/ajpheart.00916.2012
Pierce GL, Harris SA, Seals DR, Casey DP, Barlow PB, Stauss HM (2016) Estimated aortic stiffness is independently associated with cardiac baroreflex sensitivity in humans: role of ageing and habitual endurance exercise. J Hum Hypertens 30(9):513–520. https://doi.org/10.1038/jhh.2016.3
Rietjens GJ, Kuipers H, Kester AD, Keizer HA (2001) Validation of a computerized metabolic measurement system (Oxycon-Pro) during low and high intensity exercise. Int J Sports Med 22(4):291–294. https://doi.org/10.1055/s-2001-14342
Rossman MJ, LaRocca TJ, Martens CR, Seals DR (2018) Healthy lifestyle-based approaches for successful vascular aging. J Appl Physiol (1985) 125(6):1888–1900. https://doi.org/10.1152/japplphysiol.00521.2018
Seals DR, Brunt VE, Rossman MJ (2018) Keynote lecture: strategies for optimal cardiovascular aging. Am J Physiol Heart Circ Physiol 315(2):H183–H188. https://doi.org/10.1152/ajpheart.00734.2017
Seals DR, Nagy EE, Moreau KL (2019) Aerobic exercise training and vascular function with ageing in healthy men and women. J Physiol 597(19):4901–4914. https://doi.org/10.1113/JP277764
Shibata S, Fujimoto N, Hastings JL, Carrick-Ranson G, Bhella PS, Hearon CM Jr, Levine BD (2018) The effect of lifelong exercise frequency on arterial stiffness. J Physiol 596(14):2783–2795. https://doi.org/10.1113/JP275301
Shoemaker JK, Badrov MB, Al-Khazraji BK, Jackson DN (2015) Neural control of vascular function in skeletal muscle. Compr Physiol 6(1):303–329. https://doi.org/10.1002/cphy.c150004
Smith MM, Buffington CA, Hamlin RL, Devor ST (2015) Relationship between muscle sympathetic nerve activity and aortic wave reflection characteristics in aerobic- and resistance-trained subjects. Eur J Appl Physiol 115(12):2609–2619. https://doi.org/10.1007/s00421-015-3230-9
Stamatelopoulos KS, Kalpakos D, Protogerou AD, Papamichael CM, Ikonomidis I, Tsitsirikos M, Revela I, Papaioannou TG, Lekakis JP (2006) The combined effect of augmentation index and carotid intima-media thickness on cardiovascular risk in young and middle-aged men without cardiovascular disease. J Hum Hypertens 20(4):273–279. https://doi.org/10.1038/sj.jhh.1001978
Stoner L, Faulkner J, Lowe A, Lambrick DM, Young JM, Love R, Rowlands DS (2014) Should the augmentation index be normalized to heart rate? J Atheroscler Thromb 21(1):11–16. https://doi.org/10.5551/jat.20008
Swierblewska E, Hering D, Kara T, Kunicka K, Kruszewski P, Bieniaszewski L, Boutouyrie P, Somers VK, Narkiewicz K (2010) An independent relationship between muscle sympathetic nerve activity and pulse wave velocity in normal humans. J Hypertens 28(5):979–984. https://doi.org/10.1097/hjh.0b013e328336ed9a
Talbot JS, Lord RN, Wakeham DJ, Dawkins TG, Curry BA, Brown M, Lodge FM, Pugh CJA (2020) The influence of habitual endurance exercise on carotid artery strain and strain rate in young and middle-aged men. Exp Physiol. https://doi.org/10.1113/EP088384
Tan I, Spronck B, Kiat H, Barin E, Reesink KD, Delhaas T, Avolio AP, Butlin M (2016) Heart rate dependency of large artery stiffness. Hypertension 68(1):236–242. https://doi.org/10.1161/HYPERTENSIONAHA.116.07462
Tanaka H (2019) Antiaging effects of aerobic exercise on systemic arteries. Hypertension. https://doi.org/10.1161/HYPERTENSIONAHA.119.13179
Tanaka H, DeSouza CA, Seals DR (1998) Absence of age-related increase in central arterial stiffness in physically active women. Arterioscler Thromb Vasc Biol 18(1):127–132. https://doi.org/10.1161/01.atv.18.1.127
Tarumi T, Gonzales MM, Fallow B, Nualnim N, Pyron M, Tanaka H, Haley AP (2013) Central artery stiffness, neuropsychological function, and cerebral perfusion in sedentary and endurance-trained middle-aged adults. J Hypertens 31(12):2400–2409. https://doi.org/10.1097/HJH.0b013e328364decc
Tarumi T, Yamabe T, Fukuie M, Kimura R, Zhu DC, Ohyama-Byun K, Maeda S, Sugawara J (2021) Proximal aortic compliance in young male endurance athletes: an MRI Study. Med Sci Sports Exerc 53(3):543–550. https://doi.org/10.1249/MSS.0000000000002508
Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, Heffernan KS, Lakatta EG, McEniery CM, Mitchell GF, Najjar SS, Nichols WW, Urbina EM, Weber T, American Heart Association Council on H (2015) Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American Heart Association. Hypertension 66(3):698–722. https://doi.org/10.1161/HYP.0000000000000033
Vaitkevicius PV, Fleg JL, Engel JH, O’Connor FC, Wright JG, Lakatta LE, Yin FC, Lakatta EG (1993) Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation 88(4 Pt 1):1456–1462. https://doi.org/10.1161/01.cir.88.4.1456
Van Bortel LM, Laurent S, Boutouyrie P, Chowienczyk P, Cruickshank JK, De Backer T, Filipovsky J, Huybrechts S, Mattace-Raso FU, Protogerou AD, Schillaci G, Segers P, Vermeersch S, Weber T, Artery S, European Society of Hypertension Working Group on Vascular S, Function, European Network for Noninvasive Investigation of Large A (2012) Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens 30(3):445–448. https://doi.org/10.1097/HJH.0b013e32834fa8b0
Van Bortel LM, Segers P, De Backer T (2020) Misconceptions about arterial stiffness may lead to erroneous conclusions. Am J Hypertens 33(5):402–404. https://doi.org/10.1093/ajh/hpaa017
Vlachopoulos C, Aznaouridis K, O’Rourke MF, Safar ME, Baou K, Stefanadis C (2010) Prediction of cardiovascular events and all-cause mortality with central haemodynamics: a systematic review and meta-analysis. Eur Heart J 31(15):1865–1871. https://doi.org/10.1093/eurheartj/ehq024
Wakeham DJ, Lord RN, Talbot JS, Lodge FM, Curry BA, Dawkins TG, Simpson LL, Shave RE, Pugh CJA, Moore JP (2019) Upward resetting of the vascular sympathetic baroreflex in middle-aged male runners. Am J Physiol Heart Circ Physiol 317(1):H181–H189. https://doi.org/10.1152/ajpheart.00106.2019
Wang KL, Cheng HM, Sung SH, Chuang SY, Li CH, Spurgeon HA, Ting CT, Najjar SS, Lakatta EG, Yin FC, Chou P, Chen CH (2010) Wave reflection and arterial stiffness in the prediction of 15-year all-cause and cardiovascular mortalities: a community-based study. Hypertension 55(3):799–805. https://doi.org/10.1161/HYPERTENSIONAHA.109.139964
Wilkinson IB, MacCallum H, Flint L, Cockcroft JR, Newby DE, Webb DJ (2000) The influence of heart rate on augmentation index and central arterial pressure in humans. J Physiol 525(Pt 1):263–270. https://doi.org/10.1111/j.1469-7793.2000.t01-1-00263.x
Wilkinson IB, Mohammad NH, Tyrrell S, Hall IR, Webb DJ, Paul VE, Levy T, Cockcroft JR (2002) Heart rate dependency of pulse pressure amplification and arterial stiffness. Am J Hypertens 15(1 Pt 1):24–30. https://doi.org/10.1016/s0895-7061(01)02252-x
Yan H, Ranadive SM, Heffernan KS, Lane AD, Kappus RM, Cook MD, Wu PT, Sun P, Harvey IS, Woods JA, Wilund KR, Fernhall B (2014) Hemodynamic and arterial stiffness differences between African-Americans and Caucasians after maximal exercise. Am J Physiol Heart Circ Physiol 306(1):H60-68. https://doi.org/10.1152/ajpheart.00710.2013
Acknowledgements
We are grateful to the men who gave up their time freely to participate in this study; and, to Zavia Penn and Megan Brown for their valuable contributions to data collection.
Funding
DJW was supported by a PhD studentship from the School of Sport and Health Sciences, Cardiff Metropolitan University. LLS was supported by a PhD studentship from the School of Sport Health and Exercise Sciences, Bangor University.
Author information
Authors and Affiliations
Contributions
All testing was completed at the Cardiff School of Sport and Health Sciences, Cardiff Metropolitan University, Cardiff, Wales, UK. DJW, CJP, RS, and JPM contributed to conception and design of the work and acquisition, analysis and interpretation of the data and writing of the manuscript. RNL, JST, FML, BAC, TGD and LLS, contributed to acquisition, analysis and interpretation of the data and critically revised the manuscript. All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons included as an author qualify for authorship, and all those who qualify for authorship are listed.
Corresponding author
Ethics declarations
Conflict of interest
Not applicable.
Additional information
Communicated by I. Mark Olfert.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wakeham, D.J., Dawkins, T.G., Lord, R.N. et al. Aortic haemodynamics: the effects of habitual endurance exercise, age and muscle sympathetic vasomotor outflow in healthy men. Eur J Appl Physiol 122, 801–813 (2022). https://doi.org/10.1007/s00421-021-04883-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-021-04883-2