Abstract
Background and objective
To identify the most responsive and sensitive clinical outcome measures in GNE myopathy.
Methods
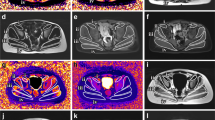
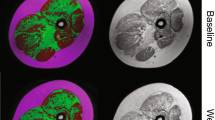
ClinBio-GNE is a natural history study in GNE myopathy. Patients were assessed prospectively by clinical, functional and quantitative nuclear magnetic resonance imaging (qNMRI) evaluations. Strength and functional tests included Myogrip, Myopinch, MoviPlate and Brooke assessments for upper limb and the 6-min walk distance for lower limb. qNMRI was performed for determining the degree of fatty infiltration and trophicity in leg, thigh, forearm and hand skeletal muscles. Ten GNE myopathy patients were included. Three patients were non-ambulant. Age and gender-matched healthy subjects were used as controls.
Results
Fatty infiltration and contractile cross-sectional area changed inversely and significantly in lower distal limbs and in proximal lower and distal upper limbs over 1 year. qNMRI indices and functional assessment results were strongly correlated.
Conclusions
Even in a limited number of patients, qNMRI could detect a significant change over a 1-year period in GNE myopathy, which suggests that qNMRI could constitute a surrogate endpoint in this slowly progressive disease. Quantitative NMRI outcome measures can monitor intramuscular fat accumulation with high responsiveness. Longer follow-up should improve our understanding of GNE myopathy evolution and also lead to the identification of non-invasive outcome measures with the highest discriminant power for upcoming clinical trials.



Similar content being viewed by others
References
Argov Z, Caraco Y, Lau H, Pestronk A, Shieh PB, Skrinar A, Koutsoukos T, Ahmed R, Martinisi J, Kakkis E (2016) Aceneuramic acid extended release administration maintains upper limb muscle strength in a 48-week study of subjects with GNE myopathy: results from a phase 2, randomized, controlled study. J Neuromuscular Dis 3:49–66
Argov Z, Yarom R (1984) "Rimmed vacuole myopathy" sparing the quadriceps. A unique disorder in Iranian Jews. J Neurol Sci 64:33–43
Azzabou N, Loureiro de Sousa P, Caldas E, Carlier PG (2015) Validation of a generic approach to muscle water T2 determination at 3T in fat-infiltrated skeletal muscle. J Magn Reson Imaging 41:645–653
Boyden SE, Duncan AR, Estrella EA, Lidov HG, Mahoney LJ, Katz JS, Kunkel LM, Kang PB (2011) Molecular diagnosis of hereditary inclusion body myopathy by linkage analysis and identification of a novel splice site mutation in GNE. BMC Med Genet 12:87
Broccolini A, Gidaro T, Morosetti R, Mirabella M (2009) Hereditary inclusion-body myopathy: clues on pathogenesis and possible therapy. Muscle Nerve 40:340–349
Broccolini A, Gidaro T, Tasca G, Morosetti R, Rodolico C, Ricci E, Mirabella M (2010) Analysis of NCAM helps identify unusual phenotypes of hereditary inclusion-body myopathy. Neurology 75:265–272
Brooke MH, Griggs RC, Mendell JR, Fenichel GM, Shumate JB, Pellegrino RJ (1981) Clinical trial in Duchenne dystrophy. I. The design of the protocol. Muscle Nerve 4:186–197
Carlier PG, Azzabou N, de Sousa PL, Hicks A, Boisserie JM, Amadon A, Carlier RY, Wary C, Orlikowski D, Laforet P (2015) Skeletal muscle quantitative nuclear magnetic resonance imaging follow-up of adult Pompe patients. J Inherit Metab Dis 38:565–572
Carlier PG, Marty B, Scheidegger O, Loureiro de Sousa P, Baudin PY, Snezhko E, Vlodavets D (2016) Skeletal muscle quantitative nuclear magnetic resonance imaging and spectroscopy as an outcome measure for clinical trials. J Neuromuscular Dis 3:1–28
Celeste FV, Vilboux T, Ciccone C, de Dios JK, Malicdan MC, Leoyklang P, McKew JC, Gahl WA, Carrillo-Carrasco N, Huizing M (2014) Mutation update for GNE gene variants associated with GNE myopathy. Hum Mutat 35:915–926
Chaouch A, Brennan KM, Hudson J, Longman C, McConville J, Morrison PJ, Farrugia ME, Petty R, Stewart W, Norwood F, Horvath R, Chinnery PF, Costigan D, Winer J, Polvikoski T, Healy E, Sarkozy A, Evangelista T, Pogoryelova O, Eagle M, Bushby K, Straub V, Lochmuller H (2014) Two recurrent mutations are associated with GNE myopathy in the North of Britain. J Neurol Neurosurg Psychiatry 85:1359–1365
Cho A, Christine M, Malicdan V, Miyakawa M, Nonaka I, Nishino I, Noguchi S (2017) Sialic acid deficiency is associated with oxidative stress leading to muscle atrophy and weakness in GNE myopathy. Hum Mol Genet 26:3081–3093
Chu CC, Kuo HC, Yeh TH, Ro LS, Chen SR, Huang CC (2007) Heterozygous mutations affecting the epimerase domain of the GNE gene causing distal myopathy with rimmed vacuoles in a Taiwanese family. Clin Neurol Neurosurg 109:250–256
de Dios JK, Shrader JA, Joe GO, McClean JC, Williams K, Evers R, Malicdan MC, Ciccone C, Mankodi A, Huizing M, McKew JC, Bluemke DA, Gahl WA, Carrillo-Carrasco N (2014) Atypical presentation of GNE myopathy with asymmetric hand weakness. Neuromuscular Disord 24:1063–1067
Eisenberg I, Avidan N, Potikha T, Hochner H, Chen M, Olender T, Barash M, Shemesh M, Sadeh M, Grabov-Nardini G, Shmilevich I, Friedmann A, Karpati G, Bradley WG, Baumbach L, Lancet D, Asher EB, Beckmann JS, Argov Z, Mitrani-Rosenbaum S (2001) The UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase gene is mutated in recessive hereditary inclusion body myopathy. Nat Genet 29:83–87
Haghighi A, Nafissi S, Qurashi A, Tan Z, Shamshiri H, Nilipour Y, Haghighi A, Desnick RJ, Kornreich R (2016) Genetics of GNE myopathy in the non-Jewish Persian population. Eur J Hum Genet 24:243–251
Hogrel JY, Wary C, Moraux A, Azzabou N, Decostre V, Ollivier G, Canal A, Lilien C, Ledoux I, Annoussamy M, Reguiba N, Gidaro T, Le Moing AG, Cardas R, Voit T, Carlier PG, Servais L (2016) Longitudinal functional and NMR assessment of upper limbs in Duchenne muscular dystrophy. Neurology
Huizing M, Carrillo-Carrasco N, Malicdan MC, Noguchi S, Gahl WA, Mitrani-Rosenbaum S, Argov Z, Nishino I (2014) GNE myopathy: new name and new mutation nomenclature. Neuromuscular Disord 24:387–389
Kan HE, Scheenen TW, Wohlgemuth M, Klomp DW, van Loosbroek-Wagenmans I, Padberg GW, Heerschap A (2009) Quantitative MR imaging of individual muscle involvement in facioscapulohumeral muscular dystrophy. Neuromuscular Disord 19:357–362
Kurochkina N, Yardeni T, Huizing M (2010) Molecular modeling of the bifunctional enzyme UDP-GlcNAc 2-epimerase/ManNAc kinase and predictions of structural effects of mutations associated with HIBM and sialuria. Glycobiology 20:322–337
Lochmuller H, Behin A, Caraco Y, Lau H, Mirabella M, Tournev I, Tarnopolsky M, Pogoryelova O, Woods C, Lai A, Shah J, Koutsoukos T, Skrinar A, Mansbach H, Kakkis E, Mozaffar T (2019) A phase 3 randomized study evaluating sialic acid extended-release for GNE myopathy. Neurology 92:e2109–e2117
Lokken N, Hedermann G, Thomsen C, Vissing J (2016) Contractile properties are disrupted in Becker muscular dystrophy, but not in limb girdle type 2I. Ann Neurol 80:466–471
Malicdan MC, Noguchi S, Hayashi YK, Nonaka I, Nishino I (2009) Prophylactic treatment with sialic acid metabolites precludes the development of the myopathic phenotype in the DMRV-hIBM mouse model. Nat Med 15:690–695
Mori-Yoshimura M, Oya Y, Yajima H, Yonemoto N, Kobayashi Y, Hayashi YK, Noguchi S, Nishino I, Murata M (2014) GNE myopathy: a prospective natural history study of disease progression. Neuromuscular Disord 24:380–386
Morrow JM, Sinclair CD, Fischmann A, Machado PM, Reilly MM, Yousry TA, Thornton JS, Hanna MG (2016) MRI biomarker assessment of neuromuscular disease progression: a prospective observational cohort study. Lancet Neurol 15:65–77
Mul K, Vincenten SCC, Voermans NC, Lemmers R, van der Vliet PJ, van der Maarel SM, Padberg GW, Horlings CGC, van Engelen BGM (2017) Adding quantitative muscle MRI to the FSHD clinical trial toolbox. Neurology
Nemunaitis G, Jay CM, Maples PB, Gahl WA, Huizing M, Yardeni T, Tong AW, Phadke AP, Pappen BO, Bedell C, Allen H, Hernandez C, Templeton NS, Kuhn J, Senzer N, Nemunaitis J (2011) Hereditary inclusion body myopathy: single patient response to intravenous dosing of GNE gene lipoplex. Hum Gene Ther 22:1331–1341
Nishino I, Carrillo-Carrasco N, Argov Z (2015) GNE myopathy: current update and future therapy. J Neurol Neurosurg Psychiatry 86:385–392
Nonaka I, Noguchi S, Nishino I (2005) Distal myopathy with rimmed vacuoles and hereditary inclusion body myopathy. Curr Neurol Neurosci Rep 5:61–65
Ricci E, Broccolini A, Gidaro T, Morosetti R, Gliubizzi C, Frusciante R, Di Lella GM, Tonali PA, Mirabella M (2006) NCAM is hyposialylated in hereditary inclusion body myopathy due to GNE mutations. Neurology 66:755–758
Seferian AM, Moraux A, Annoussamy M, Canal A, Decostre V, Diebate O, Le Moing AG, Gidaro T, Deconinck N, Van Parys F, Vereecke W, Wittevrongel S, Mayer M, Maincent K, Desguerre I, Thémar-Noël C, Cuisset JM, Tiffreau V, Denis S, Jousten V, Quijano-Roy S, Voit T, Hogrel JY, Servais L (2015) Upper limb strength and function changes during a one-year follow-up in non-ambulant patients with Duchenne Muscular Dystrophy: an observational multicenter trial. PLoS ONE 10:e0113999
Seferian AM, Moraux A, Canal A, Decostre V, Diebate O, Le Moing AG, Gidaro T, Deconinck N, Van Parys F, Vereecke W, Wittevrongel S, Annoussamy M, Mayer M, Maincent K, Cuisset JM, Tiffreau V, Denis S, Jousten V, Quijano-Roy S, Voit T, Hogrel JY, Servais L (2015) Upper limb evaluation and one-year follow up of non-ambulant patients with spinal muscular atrophy: an observational multicenter trial. PLoS ONE 10:e0121799
Servais L, Deconinck N, Moraux A, Benali M, Canal A, Van Parys F, Vereecke W, Wittevrongel S, Mayer M, Desguerre I, Maincent K, Themar-Noel C, Quijano-Roy S, Serari N, Voit T, Hogrel JY (2013) Innovative methods to assess upper limb strength and function in non-ambulant Duchenne patients. Neuromuscular Disord 23:139–148
Sparks S, Rakocevic G, Joe G, Manoli I, Shrader J, Harris-Love M, Sonies B, Ciccone C, Dorward H, Krasnewich D, Huizing M, Dalakas MC, Gahl WA (2007) Intravenous immune globulin in hereditary inclusion body myopathy: a pilot study. BMC Neurol 7:3
Tasca G, Ricci E, Monforte M, Laschena F, Ottaviani P, Rodolico C, Barca E, Silvestri G, Iannaccone E, Mirabella M, Broccolini A (2012) Muscle imaging findings in GNE myopathy. J Neurol 259:1358–1365
Toriumi Y, Takusa Y, Uchiyama A, Kimura M, Sejima H, Yamaguchi S, Eda I, Nishino I, Nonaka I (2006) Distal myopathy with rimmed vacuoles in a case of opercular syndrome. Brain Dev 28:458–461
Voermans NC, Guillard M, Doedee R, Lammens M, Huizing M, Padberg GW, Wevers RA, van Engelen BG, Lefeber DJ (2010) Clinical features, lectin staining, and a novel GNE frameshift mutation in hereditary inclusion body myopathy. Clin Neuropathol 29:71–77
Willcocks RJ, Rooney WD, Triplett WT, Forbes SC, Lott DJ, Senesac CR, Daniels MJ, Wang DJ, Harrington AT, Tennekoon GI, Russman BS, Finanger EL, Byrne BJ, Finkel RS, Walter GA, Sweeney HL, Vandenborne K (2016) Multicenter prospective longitudinal study of magnetic resonance biomarkers in a large duchenne muscular dystrophy cohort. Ann Neurol 79:535–547
Willis TA, Hollingsworth KG, Coombs A, Sveen ML, Andersen S, Stojkovic T, Eagle M, Mayhew A, de Sousa PL, Dewar L, Morrow JM, Sinclair CD, Thornton JS, Bushby K, Lochmuller H, Hanna MG, Hogrel JY, Carlier PG, Vissing J, Straub V (2013) Quantitative muscle MRI as an assessment tool for monitoring disease progression in LGMD2I: a multicentre longitudinal study. PLoS ONE 8:e70993
Zhao J, Wang Z, Hong D, Lv H, Zhang W, Chen J, Yuan Y (2015) Mutational spectrum and clinical features in 35 unrelated mainland Chinese patients with GNE myopathy. J Neurol Sci 354:21–26
Acknowledgements
The authors thank the patients and their families for participating in the study. We acknowledge the dedicated work of Dominique Duchêne, Aurelie Chabanon and Gwenn Ollivier for help with data collection and protocol organization.
Author information
Authors and Affiliations
Contributions
Conception and design of the study: TG, PC, LS. NMR data acquisition, processing and analysis: HR, JLL, EA, PYB, BM. Clinical evaluation and follow-up of patients: TG, AB, FT, MV. Data interpretation: TG, HR, MA, J-YH, PC, LS. Statistical analysis: TG. Drafting the manuscript: TG, HR, LS. Study Supervision: JYH, PC, LS. Revising and approving the manuscript: all authors.
Corresponding author
Ethics declarations
Conflicts of interest
T. Gidaro and A. Behin have served on scientific advisory boards for Ultragenyx. J-Y Hogrel is co-inventor of the MyoGrip, MyoPinch and MoviPlate. L. Servais is co-inventor of the MoviPlate. H. Reyngoudt, J. Le Louër, F. Toumi, E. Villeret M., Araujo, P-Y. Baudin, B. Marty, M. Annoussamy and P. Carlier report no disclosures relevant to the manuscript.
Rights and permissions
About this article
Cite this article
Gidaro, T., Reyngoudt, H., Le Louër, J. et al. Quantitative nuclear magnetic resonance imaging detects subclinical changes over 1 year in skeletal muscle of GNE myopathy. J Neurol 267, 228–238 (2020). https://doi.org/10.1007/s00415-019-09569-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-019-09569-6