Abstract
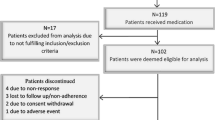
Sustained-release fampridine (fampridine-SR) improves gait velocity and self-perceived capacities in people with multiple sclerosis (MS). However, little is known about the treatment’s effect on temporospatial gait parameters, walking endurance, general fatigue, hand function and quality of life (QoL). We therefore sought to evaluate these parameters in a real-world setting: 120 consecutive, eligible patients with MS were evaluated at baseline (D0) and after two weeks (D14) of fampridine-SR. Lastly, D14 responders were again evaluated after three months (M3). Response to treatment was defined as a 15 % improvement in at least one of the following tests: the Timed 25-Foot-Walk (T25FW), the 2-min walk test (2MWT) and the Multiple Sclerosis Walking Scale (MSWS-12). Eighty-three patients (74 %) were found to be responders. The response rate was lower when assessed as a 20 % improvement in the T25FW (50.9 %), and this difference was particularly marked for fast-walking subjects (i.e. T25FW <8 s at baseline). Responders displayed mean improvements (at D14 and M3, respectively) of 34.5 and 35.5 % in the T25FW, 39 and 36.7 % in the 2MWT and 19 and 11.6 % in the MSWS-12. The increase in gait velocity was due to both a higher cadence and a greater step length. Responders showed also significant, lasting reductions in fatigue (visual analogue scale and the Fatigue Severity Scale; p < 10−4 at D14 and <0.01 at M3) and significant, lasting improvements in hand function (9 Hole Peg Test; p < 0.05) and QoL (SF-12; p < 0.01). In conclusion, several MS-induced symptoms other than gait velocity may be improved by fampridine-SR, even if this remains to be more specifically evaluated in future studies.


Similar content being viewed by others
References
Krupp L (2006) Fatigue is intrinsic to multiple sclerosis (MS) and is the most commonly reported symptom of the disease. Mult Scler 12:367–368
Giovannoni G (2006) Multiple sclerosis related fatigue. J Neurol Neurosurg Psychiatry 77:2–3
Scalfari A, Neuhaus A, Degenhardt A et al (2010) The natural history of multiple sclerosis: a geographically based study 10: relapses and long-term disability. Brain J Neurol 133:1914–1929
Lamers I, Kerkhofs L, Raats J et al (2013) Perceived and actual arm performance in multiple sclerosis: relationship with clinical tests according to hand dominance. Mult Scler 19:1341–1348
Miller DM, Allen R (2010) Quality of life in multiple sclerosis: determinants, measurement, and use in clinical practice. Curr Neurol Neurosci Rep 10:397–406
Kaji R, Sumner AJ (1988) Effects of 4-aminopyridine in experimental CNS demyelination. Neurology 38:1884–1887
Goodman AD, Brown TR, Edwards KR et al (2010) A phase 3 trial of extended release oral dalfampridine in multiple sclerosis. Ann Neurol 68:494–502
Goodman AD, Brown TR, Krupp LB et al (2009) Sustained-release oral fampridine in multiple sclerosis: a randomised, double-blind, controlled trial. Lancet 373:732–738
Goodman AD, Bethoux F, Brown TR et al (2015) Long-term safety and efficacy of dalfampridine for walking impairment in patients with multiple sclerosis: results of open-label extensions of two phase 3 clinical trials. Mult Scler, Epub
Rabadi MH, Kreymborg K, Vincent AS (2013) Sustained-release fampridine (4-aminopyridine) in multiple sclerosis: efficacy and impact on motor function. Drugs RD 13:175–181
Ruck T, Bittner S, Simon OJ et al (2014) Long-term effects of dalfampridine in patients with multiple sclerosis. J Neurol Sci 337:18–24
Prugger M, Berger T (2013) Assessing the long-term clinical benefit of prolonged-release fampridine tablets in a real-world setting: a review of 67 cases. Patient Relat Outcome Meas 4:75–85
Jensen H, Ravnborg M, Mamoei S et al (2014) Changes in cognition, arm function and lower body function after Slow-Release Fampridine treatment. Mult Scler 20:1872–1880
McDonald WI, Compston A, Edan G et al (2001) Recommended diagnostic criteria for multiple sclerosis: guidelines from the international panel on the diagnosis of multiple sclerosis. Ann Neurol 50:121–127
Fischer JS, Rudick RA, Cutter GR, Reingold SC (1999) The multiple sclerosis functional composite measure (MSFC): an integrated approach to MS clinical outcome assessment. National MS society clinical outcomes assessment task force. Mult Scler 5:244–250
Gijbels D, Eijnde BO, Feys P (2011) Comparison of the 2- and 6-min walk test in multiple sclerosis. Mult Scler 17:1269–1272
Phan-Ba R, Calay P, Grodent P et al (2012) Motor fatigue measurement by distance-induced slow down of walking speed in multiple sclerosis. PLoS One 7:e34744
Givon U, Zeilig G, Achiron A (2009) Gait analysis in multiple sclerosis: characterization of temporal-spatial parameters using GAITRite functional ambulation system. Gait Posture 29:138–142
Hobart JC, Riazi A, Lamping DL et al (2003) Measuring the impact of MS on walking ability: the 12-Item MS Walking Scale (MSWS-12). Neurology 60:31–36
Goodkin DE, Hertsgaard D, Seminary J (1988) Upper extremity function in multiple sclerosis: improving assessment sensitivity with box-and-block and nine-hole peg tests. Arch Phys Med Rehabil 69:850–854
Lwin CTT, Bishay M, Platts RG et al (2003) The assessment of fatigue in primary Sjogren’s syndrome. Scand J Rheumatol 32:33–37
Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD (1989) The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol 46:1121–1123
Ware J Jr, Kosinski M, Keller SD (1996) A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care 34:220–233
Coleman CI, Sobieraj DM, Marinucci LN (2012) Minimally important clinical difference of the Timed 25-Foot Walk Test: results from a randomized controlled trial in patients with multiple sclerosis. Curr Med Res Opin 28:49–56
Goodman AD, Brown TR, Cohen JA et al (2008) Dose comparison trial of sustained-release fampridine in multiple sclerosis. Neurology 71:1134–1141
Goodman AD, Cohen JA, Cross A et al (2007) Fampridine-SR in multiple sclerosis: a randomized, double-blind, placebo-controlled, dose-ranging study. Mult Scler 13:357–368
Cohen JA, Cutter GR, Fischer JS et al (2001) Use of the multiple sclerosis functional composite as an outcome measure in a phase 3 clinical trial. Arch Neurol 58:961–967
Solari A, Radice D, Manneschi L et al (2005) The multiple sclerosis functional composite: different practice effects in the three test components. J Neurol Sci 228:71–74
Conflicts of interest
Professor Vermersch serves on scientific advisory boards for Biogen Idec and has received travel funding. Drs Zephir and Outteryck have received travel funding from Biogen Idec.
Ethical standards
The study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its amendments. All patients provided their written, informed consent to participation.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Allart, E., Benoit, A., Blanchard-Dauphin, A. et al. Sustained-released fampridine in multiple sclerosis: effects on gait parameters, arm function, fatigue, and quality of life. J Neurol 262, 1936–1945 (2015). https://doi.org/10.1007/s00415-015-7797-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-015-7797-1