Abstract
Purpose
Based on the reported tocolytic action of the hormone relaxin (RLX) in rodents, locally produced in reproductive tissues and the corpus luteum in mammals, the present study aimed to evaluate the influence of RLX on contraction-mediating cyclooxygenases-1 and -2 (COX) and the contractile prostaglandin PGE2 in human myometrial and decidual cells. Primary cultured cells were obtained from uteri and placentas of term and preterm women undergoing elective caesarean section.
Methods
In vitro culture of primary myometrial and decidual cells, immunocytochemistry, reverse transcription and real-time PCR, Western blot, ELISA.
Results
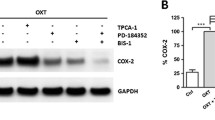
We demonstrate for the first time an activating effect of RLX for human COX-1 and COX-2 in primary myometrial and decidual cells in vitro.
Conclusions
These effects might potentially contribute to birth-associated induction of contractions in vivo.



Similar content being viewed by others
References
WHO The world health report 2000
Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, Chaiworapongsa T, Mazor M (2006) The preterm parturition syndrome. BJOG 113(Suppl 3):17–42. doi:10.1111/j.1471-0528.2006.01120.x
Simhan HN, Caritis SN (2007) Prevention of preterm delivery. N Engl J Med 357:477–487. doi:10.1056/NEJMra050435
Hisaw HL (1926) Experimental relaxation of the pubic ligament of guinea pig. Proc Soc Exp Biol Med 23:661–663
Bani D (1997) Relaxin: a pleiotropic hormone. Gen Pharmacol 28:13–22. doi:10.1016/S0306-3623(96)00171-1
Wilkinson TN, Speed TP, Tregear GW, Bathgate RAD (2005) Evolution of the relaxin-like peptide family. BMC Evol Biol 5:14–30. doi:10.1186/1471-2148-5-14
Hsu SY, Nakabayashi K, Nishi S, Kumagai J, Kudo M, Sherwood OD, Hsueh AJ (2002) Activation of orphan receptors by the hormone relaxin. Science 295:671–674. doi:10.1126/science.1065654
Bathgate RA, Ivell R, Sanborn BM, Sherwood OD, Summers RJ (2006) International Union of Pharmacology LVII: recommendations for the nomenclature of receptors for relaxin family peptides. Pharmacol Rev 58:7–31. doi:10.1124/pr.58.1.9
Crawford RJ, Hudson P, Shine J, Niall HD, Eddy RL, Shows TB (1984) Two human relaxin genes are on chromosome 9. EMBO J 3:2341–2345
Bathgate RAD, Samuel CS, Burazin TCD, Layfield S, Claasz AA, Reytomas IGT (2002) Human relaxin gene 3 (H3) and the equivalent mouse relaxin (M3) gene—novel members of the relaxin peptide family. J Biol Chem 277:1148–1157. doi:10.1074/jbc.M107882200
Bullesbach EE, Schwabe C (2000) The relaxin receptor-binding site geometry suggests a novel gripping mode of interaction. J Biol Chem 275:35276–35280. doi:10.1074/jbc.M005728200
Hansell DJ, Bryant-Greenwood GD, Greenwood FC (1991) Expression of the human relaxin H1 gene in the deciduas, trophoblast, and prostate. J Clin Endocrinol Metab 72:899–904
Liu C, Eriste E, Sutton S, Chen J, Roland B, Kuei C, Farmer N, Jörnvall H, Sillard R, Lovenberg TW (2003) Identification of relaxin-3/INSL7 as an endogenous ligand for the orphan G-protein-coupled receptor GPCR135. J Biol Chem 278:50754–50764. doi:10.1074/jbc.M308995200
Ivell R, Einspanier A (2002) Relaxin peptides are new global players. Trends Endocrinol Metab 13:343–348. doi:10.1016/S1043-2760(02)00664-1
Scott DJ, Fu P, Shen P-J, Gundlach A, Layfield S, Riesewijk A, Tregear GW, Bathgate RAD (2005) Characterisation of the rat INSL3 receptor. Ann N Y Acad Sci 1041:13–16. doi:10.1196/annals.1282.003
Osa T, Inoue H, Okabe K (1991) Effects of porcine relaxin on contraction, membrane response and cyclicAMP content in rat myometrium in comparison with the effects of isoprenaline and forskolin. Br J Pharmacol 104:950–960
Downing SJ, Hollingsworth M (1993) Action of relaxin on uterine contractions. J Reprod Fertil 99:275–282. doi:10.1530/jrf.0.0990275
Weiss G, Goldsmith LT, Sachdev R, Von Hagen S, Lederer K (1993) Elevated first-trimester serum relaxin concentrations in pregnant women following ovarian stimulation predict prematurity risk and preterm delivery. Obstet Gynecol 82:821–828
Bell RJ, Eddie LW, Lester AR, Wood EC, Johnston PD, Niall HD (1988) Antenatal serum levels of relaxin in patients having preterm labour. Br J Obstet Gynaecol 95:1264–1267
Eddie LW, Bell RJ, Lester A, Geier M, Bennett G, Johnston PD, Niall HD (1986) Radioimmunoassay of relaxin in pregnancy with an analogue of human relaxin. Lancet 1:1344–1346. doi:10.1016/S0140-6736(86)91662-4
Anwer K, Hovington JA, Sanborn BM (1989) Antagonism of contractants and relaxants at the level of intracellular calcium and phosphoinositide turnover in the rat uterus. Endocrinology 124:2995–3002
Sanborn BM (2001) Hormones and calcium: mechanisms controlling uterine smooth muscle contractile activity. Exp Physiol 86:223–237. doi:10.1113/eph8602179
Barata H, Thompson M, Zielinska W, Han YS, Mantilla CB, Prakash YS et al (2004) The role of cyclic-ADP-ribose-signaling pathway in oxytocin induced Ca2+ transients in human myometrium cells. Endocrinology 145:881–889. doi:10.1210/en.2003-0774
Sanborn BM, Qian A, Ku CY, Wen Y, Anwer K, Monga M (1995) Mechanisms regulating oxytocin receptor coupling to phospholipase C in rat and human myometrium. Adv Exp Med Biol 395:469–479
Zhong M, Ku CY, Sanborn BM (2005) Pathways used by relaxin to regulate myometrial phospholipase C. Ann NY Acad Sci 1041:300–304. doi:10.1196/annals.1282.045
Hsu CJ, Sanborn BM (1986) Relaxin treatment alters the kinetic properties of myosin light chain kinetic properties of MLCK activity in rat myometrial cells in culture. Endocrinology 118:499–505
MacLennan AH, Grant P, Bryant-Greenwood G (1995) H-RLX-1 in vitro response of human and pig myometrium. J Reprod Med 40(10):703–706
Garavito RM, DeWitt DL (1999) The cyclooxygenase isoforms: structural insights into the conversion of arachidonic acid to prostaglandins. Biochim Biophys Acta 1441(2–3):278–287
Fitzpatrick FA (2004) Cyclooxygenase enzymes: regulation and function. Curr Pharm Des 10(6):577–588. doi:10.2174/1381612043453144
Reese J, Paria BC, Brown N, Zhao X, Morrow JD, Dey SK (2000) Coordinated regulation of fetal and maternal prostaglandins directs successful birth and postnatal adaptation in the mouse. Proc Natl Acad Sci USA 97(17):9759–9764. doi:10.1073/pnas.97.17.9759
Rioux N, Castonguay A (2000) The induction of cyclooxygenase-1 by a tobacco carcinogen in U937 human macrophages is correlated to the activation of NF-kappaB. Carcinogenesis 21(9):1745–1751. doi:10.1093/carcin/21.9.1745
Rauk PN, Friebe-Hoffmann U (2000) Interleukin-1β down-regulates the oxytocin receptor in cultured uterine smooth muscle cells. Am J Reprod Immunol 43:85–91. doi:10.1111/j.8755-8920.2000.430204.x
Friebe-Hoffmann U, Baston DM, Chiao JP, Winebrenner LD, Krüssel JS, Hoffmann TK, Hirchenhain J, Rauk PN (2007) The effect of relaxin on the oxytocin receptor in human uterine smooth muscle cells. Regul Pept 138:74–81. doi:10.1016/j.regpep.2006.08.004
Delvin EE, Arabian A, Glorieux FH, Mamer OA (1985) In vitro metabolism of 25-hydroxy-cholecalciferol by isolated cells from human decidua. J Clin Endocrinol Metab 60(5):880–885
Longo M, Jain V, Vedernikov YP, Garfield RE, Saade GR (2003) Effects of recombinant human relaxin on pregnant rat uterine artery and myometrium in vitro. Am J Obstet Gynecol 188(6):1468–1474. doi:10.1067/mob.2003.454 (discussion 1474–1476)
Vogel I, Grønbaek H, Uldbjerg N, Forman A (2004) The influence of amphotericin B and neomycin on the effect of human relaxin-2 on foetal membranes and isolated myometrium. Basic Clin Pharmacol Toxicol 94(3):144–150
Driver PM, Kilby MD, Walker EA, Hewison M, Stewart PM (2001) Expression of 11β-hydroxysteroid dehydrogenase isozymes and corticosteroid hormone receptors in primary cultures of human trophoblast and placental bed biopsies. Mol Hum Reprod 7:357–363. doi:10.1093/molehr/7.4.357
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thio-cyanate–phenol–chloroform extraction. Anal Biochem 162(1):156–159. doi:10.1016/0003-2697(87)90021-2
Kienzle N, Young D, Zehntner S, Bushell G, Sculley TB (1996) DNase I treatment is a prerequisite for the amplification of cDNA from episomal-based genes. Biotechniques 20(4):612–616
Can A, Tekelioglu M, Baltaci A (1995) Expression of desmin and vimentin intermediate filaments in human-decidual cells during first trimester pregnancy. Placenta 16:261–275. doi:10.1016/0143-4004(95)90113-2
Goldsmith LT, Weiss G, Steinetz BG (1995) Relaxin and its role in pregnancy. Endocrinol Metab Clin North Am 24:171–186
Telgmann G, Gellersen B (1998) Marker genes of decidualization: activation of the decidual prolactin gene. Hum Reprod Update 4:472–479. doi:10.1093/humupd/4.5.472
Nguyen BT, Yang L, Sanborn BM, Dessauer CW (2003) Phosphoinositide 3-kinase activity is required for biphasic stimulation of cyclic adenosine 3′,5′-monophosphate by relaxin. Mol Endocrinol 17(6):1075–1084. doi:10.1210/me.2002-0284
Keirse MJ (2003) The history of tocolysis. BJOG 110(Suppl 20):94–97
Kuznetsova LA, Fedin AN, Chistiakova OV, Plesneva SA, Shpakov AO, Pertseva MN (2006) Involvement of adenylyn cyclase signaling mechanisms in relaxing effect of relaxin and insulin on the rat uterus, trachea and human myometrium. Ross Fiziol Zh Im I M Sechenova 92(7):863–871
Phaneuf S, Europe-Finner GN, Carrasco MP, Hamilton CH, López Bernal A (1995) Oxytocin signalling in human myometrium. Adv Exp Med Biol, pp 453–467
Fuchs AR, Fuchs F, Husslein P (1982) Oxytocin receptors and human parturition: a dual role for oxytocin in the initiation of labor. Science 215:1396–1398. doi:10.1126/science.6278592
Chibbar R, Miller FD, Mitchell BF (1993) Synthesis of oxytocin in amnion, chorion and decidua may influence the timing of human parturition. J Clin Invest 91:185–192. doi:10.1172/JCI116169
Gross GA, Imamura T, Luedke C, Vogt SK, Olson LM, Nelson DM, Sadovsky Y, Muglia LJ (1998) Opposing actions of prostaglandins and oxytocin determine the onset of murine labor. Proc Natl Acad Sci USA 95(20):11875–11879. doi:10.1073/pnas.95.20.11875
Zhao B, Koon D, Curtis AL, Soper J, Bethin KE (2007) Identification of 9 uterine genes that are regulated during mouse pregnancy and exhibit abnormal levels in the cyclooxygenase-1 knockout mouse. Reprod Biol Endocrinol 5:28. doi:10.1186/1477-7827-5-28
Sparey C, Robson SC, Bailey J, Lyall F, Europe-Finner GN (1999) The differential expression of myometrial connexin-43, cyclooxygenase-1 and -2, and Gsa proteins in the upper and lower segments of the human uterus during pregnancy and labor. J Clin Endocrinol Metab 1999(84):1705–1710. doi:10.1210/jc.84.5.1705
Winchester SK, Imamura T, Gross GA, Muglia LM, Vogt SK, Wright J, Watanabe K, Tai HH, Muglia LJ (2002) Coordinate regulation of prostaglandin metabolism for induction of parturition in mice. Endocrinology 143(7):2593–2598. doi:10.1210/en.143.7.2593
Gupta DK, Sato TA, Keelan JA, Marvin KW, Mitchell MD (2001) Expression of prostaglandin H synthase-1 and -2 in murine intrauterine and gestational tissues from mid pregnancy until term. Prostaglandins Other Lipid Mediat 66(1):17–25. doi:10.1016/S0090-6980(01)00122-8
Molnár M, Rigó J, Romero R, Hertelendy F (1999) Oxytocin activates mitogen-activated protein kinase and up-regulates cyclooxygenase-2 and prostaglandin production in human myometrial cells. Am J Obstet Gynecol 181:42–49. doi:10.1016/S0002-9378(99)70434-5
Belt AR, Baldassare JJ, Molnár M, Romero R, Hertelendy F (1999) The nuclear factor NF-κB mediates interleukin-1β-induced expression of cycloxygenase-2 in human myometrial cells. Am J Obstet Gynecol 181:359–366. doi:10.1016/S0002-9378(99)70562-4
Rauk PN, Chiao JP (2000) Interleukin-1 stimulates human uterine prostaglandin production through induction of cyclooxygenase-2 expression. Am J Reprod Immunol 43(3):152–159. doi:10.1111/j.8755-8920.2000.430304.x
Friebe-Hoffmann U, Chiao JP, Rauk PN (2001) Effect of IL-1beta and IL-6 on oxytocin secretion in human uterine smooth muscle cells. Am J Reprod Immunol 46(3):226–231. doi:10.1034/j.1600-0897.2001.d01-6.x
Friebe-Hoffmann U, Baston DM, Hoffmann TK, Chiao JP, Rauk PN (2007) The influence of interleukin-1beta on oxytocin signalling in primary cells of human decidua. Regul Pept 142(3):78–85. doi:10.1016/j.regpep.2007.01.012
Macchiarini F, Manz MG, Palucka AK, Shultz LD (2005) Humanized mice: are we there yet? J Exp Med 202:1307–1311. doi:10.1084/jem.20051547
Acknowledgments
We thank the German Research Foundation (DFG Fr 1402/3–1) for financial support.
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baston-Büst, D.M., Hess, A.P., Hirchenhain, J. et al. A possible ambivalent role for relaxin in human myometrial and decidual cells in vitro. Arch Gynecol Obstet 280, 961–969 (2009). https://doi.org/10.1007/s00404-009-1046-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-009-1046-8