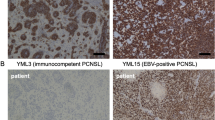
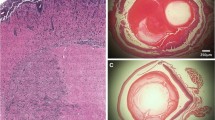
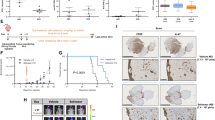
Abstract
Primary lymphoma of the central nervous system (CNS, PCNSL) is a specific diffuse large B cell lymphoma entity arising in and confined to the CNS. Despite extensive research since many decades, the pathogenetic mechanisms underlying the remarkable tropism of this peculiar malignant hematopoietic tumor remain still to be elucidated. In the present review, we summarize the present knowledge on the genotypic and phenotypic characteristics of the tumor cells of PCNSL, give an overview over deregulated molecular pathways in PCNSL and present recent progress in the field of preclinical modeling of PCNSL in mice. With regard to the phenotype, PCNSL cells resemble late germinal center exit IgM+IgD+ B cells with blocked terminal B cell differentiation. They show continued BCL6 activity in line with ongoing activity of the germinal center program. This together with the pathways deregulated by genetic alterations may foster B cell activation and brisk proliferation, which correlated with the simultaneous MYC and BCL2 overexpression characteristic for PCNSL. On the genetic level, PCNSL are characterized by ongoing aberrant somatic hypermutation that, besides the IG locus, targets the PAX5, TTF, MYC, and PIM1 genes. Moreover, PCNSL cells show impaired IG class switch due to sμ region deletions, and PRDM1 mutations. Several important pathways, i.e., the B cell receptor (BCR), the toll-like receptor, and the nuclear factor-κB pathway, are activated frequently due to genetic changes affecting genes like CD79B, SHIP, CBL, BLNK, CARD11, MALT1, BCL2, and MYD88. These changes likely foster tumor cell survival. Nevertheless, many of these features are also present in subsets of systemic DLBLC and might not be the only reasons for the peculiar tropism of PCNSL. Here, preclinical animal models that closely mimic the clinical course and neuropathology of human PCNSL may provide further insight and we discuss recent advances in this field. Such models enable us to understand the pathogenetic interaction between the malignant B cells, resident cell populations of the CNS, and the associated inflammatory infiltrate. Indeed, the immunophenotype of the CNS as well as tumor cell characteristics and intracerebral interactions may create a micromilieu particularly conducive to PCNSL that may foster aggressiveness of tumor cells and accelerate the fatal course of disease. Suitable animal models may also serve as a well-defined preclinical system and may provide a useful tool for developing new specific therapeutic strategies.




Similar content being viewed by others
References
Alldinger S, Fonfara S, Kremmer E, Baumgärtner W (2000) Up-regulation of the hyaluronate receptor CD44 in canine distemper demyelinated plaques. Acta Neuropathol 99:138–146
Baraniskin A, Kuhnhenn J, Schlegel U et al (2011) Identification of microRNAs in the cerebrospinal fluid as marker for primary diffuse large B-cell lymphoma of the central nervous system. Blood 117:3140–3146
Barrans SL, Fenton JA, Banham A, Owen RG, Jack AS (2004) Strong expression of FOXP1 identifies a distinct subset of diffuse large B-cell lymphoma (DLBCL) patients with poor outcome. Blood 104:2933–2935
Bartholomäus I, Kawakami N, Odoardi F et al (2009) Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature 462:94–98
Bashir R, Coakham H, Hochberg F (1992) Expression of LFA-1/ICAM-1 in CNS lymphomas: possible mechanism for lymphoma homing into the brain. J Neurooncol 12:103–110
Basso K, Dalla-Favera R (2010) BCL6: master regulator of the germinal center reaction and key oncogene in B cell lymphomagenesis. Adv Immunol 105:193–210
Basso K, Dalla-Favera R (2012) Roles of BCL6 in normal and transformed germinal center B cells. Immunol Rev 247:172–183
Bechmann I, Priller J, Kovac A et al (2001) Immune surveillance of mouse brain perivascular spaces by blood-borne macrophages. Eur J Neurosci 14:1651–1658
Ben Abdelwahed R, Cosette J, Donnou S et al (2013) Lymphoma B-cell responsiveness to CpG-DNA depends on the tumor microenvironment. J Exp Clin Cancer Res 32:18
Ben Abdelwahed R, Donnou S, Ouakrim H et al (2013) Preclinical study of ublituximab, a glycoengineered anti-human CD20 antibody, in murine models of primary cerebral and intraocular B-cell lymphomas. Invest Ophthalmol Vis Sci 54:3657–3665
Booman M, Douwes J, Glas AM et al (2006) Mechanisms and effects of loss of human leukocyte antigen class II expression in immune-privileged site-associated B-cell lymphoma. Clin Cancer Res 12:2698–2705
Brunn A, Montesinos-Rongen M, Strack A et al (2007) Expression pattern and cellular sources of chemokines in primary central nervous system lymphoma. Acta Neuropathol 114:271–276
Brunn A, Nagel I, Montesinos-Rongen M et al (2013) Frequent triple-hit expression of MYC, BCL2, and BCL6 in primary lymphoma of the central nervous system and absence of a favorable MYClowBCL2low subgroup may underlie the inferior prognosis as compared to systemic diffuse large B cell lymphomas. Acta Neuropathol doi. doi:10.1007/s00401-00013-01169-00407
Butler MP, Iida S, Capello D et al (2002) Alternative translocation breakpoint cluster region 5′ to BCL-6 in B-cell non-Hodgkin’s lymphoma. Cancer Res 62:4089–4094
Cady FM, O’Neill BP, Law ME et al (2008) Del(6)(q22) and BCL6 rearrangements in primary CNS lymphoma are indicators of an aggressive clinical course. J Clin Oncol 26:4814–4819
Chu LC, Eberhart CG, Grossman SA, Herman JG (2006) Epigenetic silencing of multiple genes in primary CNS lymphoma. Int J Cancer 119:2487–2491
Cobbers JM, Wolter M, Reifenberger J et al (1998) Frequent inactivation of CDKN2A and rare mutation of TP53 in PCNSL. Brain Pathol 8:263–276
Courts C, Brunn A, Montesinos-Rongen M et al (2009) Preferential expression of truncated isoforms of FOXP1 in primary central nervous system lymphoma. J Neuropathol Exp Neurol 68:972–976
Courts C, Montesinos-Rongen M, Brunn A et al (2008) Recurrent inactivation of the PRDM1 gene in primary central nervous system lymphoma. J Neuropathol Exp Neurol 67:720–727
Courts C, Montesinos-Rongen M, Martin-Subero JI et al (2007) Transcriptional profiling of the nuclear factor-kappaB pathway identifies a subgroup of primary lymphoma of the central nervous system with low BCL10 expression. J Neuropathol Exp Neurol 66:230–237
Deckert M, Brunn A, Montesinos-Rongen M, Terreni MR, Ponzoni M (2013) Primary lymphomas of the central nervous system—a diagnostic challenge. Hematol Oncol. doi:10.1002/jon.2087
Deckert M, Paulus W (2007) Malignant lymphomas. In: Louis DN, Ohgaki H, Wiestler OD, Cavenee WK (eds) WHO classification of tumors pathology ans genetics of tumours of the nervous System, 4th edn. IRAC, Lyon, pp 188–192
Deckert M, Soltek S, Geginat G et al (2001) Endogenous interleukin-10 is required for prevention of a hyperinflammatory intracerebral immune response in Listeria monocytogenes meningoencephalitis. Infect Immun 69:4561–4571
Deckert-Schlüter M, Buck C, Weiner D et al (1997) Interleukin-10 downregulates the intracerebral immune response in chronic toxoplasma encephalitis. J Neuroimmunol 76:167–176
Dominguez-Sola D, Victora GD, Ying CY et al (2012) The proto-oncogene MYC is required for selection in the germinal center and cyclic reentry. Nat Immunol 13:1083–1091
Donnou S, Galand C, Daussy C et al (2011) Immune adaptive microenvironment profiles in intracerebral and intrasplenic lymphomas share common characteristics. Clin Exp Immunol 165:329–337
Ferreri AJ, Dell’Oro S, Capello D et al (2004) Aberrant methylation in the promoter region of the reduced folate carrier gene is a potential mechanism of resistance to methotrexate in primary central nervous system lymphomas. Br J Haematol 126:657–664
Fischer L, Hummel M, Korfel A, Lenze D, Joehrens K, Thiel E (2011) Differential micro-RNA expression in primary CNS and nodal diffuse large B-cell lymphomas. Neuro Oncol 13:1090–1098
Frei K, Lins H, Fontana A (1994) Production and function of IL-10 in the central nervous system. Schweiz Arch Neurol Psychiatr 145:30–31
Frei K, Lins H, Schwerdel C, Fontana A (1994) Antigen presentation in the central nervous system. The inhibitory effect of IL-10 on MHC class II expression and production of cytokines depends on the inducing signals and the type of cell analyzed. J Immunol 152:2720–2728
Frei K, Nadal D, Pfister HW, Fontana A (1993) Listeria meningitis: identification of a cerebrospinal fluid inhibitor of macrophage listericidal function as interleukin 10. J Exp Med 178:1255–1261
Gallo P, Sivieri S, Rinaldi L et al (1994) Intrathecal synthesis of interleukin-10 (IL-10) in viral and inflammatory diseases of the central nervous system. J Neurol Sci 126:49–53
Gonzalez-Aguilar A, Idbaih A, Boisselier B et al (2012) Recurrent mutations of MYD88 and TBL1XR1 in primary central nervous system lymphomas. Clin Cancer Res 18:5203–5211
Gonzalez-Gomez P, Bello MJ, Arjona D et al (2003) CpG island methylation of tumor-related genes in three primary central nervous system lymphomas in immunocompetent patients. Cancer Genet Cytogenet 142:21–24
Green TM, Young KH, Visco C et al (2012) Immunohistochemical double-hit score is a strong predictor of outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol 30:3460–3467
Haegel H, Tölg C, Hofmann M, Ceredig R (1993) Activated mouse astrocytes and T cells express similar CD44 variants. Role of CD44 in astrocyte/T cell binding. J Cell Biol 122:1067–1077
Horn H, Ziepert M, Becher C et al (2013) MYC status in concert with BCL2 and BCL6 expression predicts outcome in diffuse large B-cell lymphoma. Blood 121:2253–2263
Ishiguro A, Suzuki Y, Inaba Y, Komiyama A, Koeffler HP, Shimbo T (1996) Production of interleukin-10 in the cerebrospinal fluid in aseptic meningitis of children. Pediatr Res 40:610–614
Jahnke K, Muldoon LL, Varallyay CG et al (2009) Efficacy and MRI of rituximab and methotrexate treatment in a nude rat model of CNS lymphoma. Neuro Oncol 11:503–513
Jin Kim K, Kanellopoulos-Langevin C, Merwin RM, Sachs DH, Asofsky R (1979) Establisment and characterization of BALB/c lymphoma lines with B cell properties. J Immunol 122:549–554
Johnson NA, Slack GW, Savage KJ et al (2012) Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol 30:3452–3459
Kadoch C, Dinca EB, Voicu R et al (2009) Pathologic correlates of primary central nervous system lymphoma defined in an orthotopic xenograft model. Clin Cancer Res 15:1989–1997
Kim N, Martin TE, Simon MC, Storb U (2003) The transcription factor Spi-B is not required for somatic hypermutation. Mol Immunol 39:577–583
Klein U, Casola S, Cattoretti G et al (2006) Transcription factor IRF4 controls plasma cell differentiation and class-switch recombination. Nat Immunol 7:773–782
Kluin P, Deckert M, Ferry JA (2008) Primary diffuse large B-cell lymphoma of the CNS. In: Swerdlow SH, Campo E, Harris NL et al (eds) WHO classification of tumours of haematopoietic and lymphoid tissues. IARC, Lyon, pp 240–241
Kornelisse RF, Savelkoul HF, Mulder PH et al (1996) Interleukin-10 and soluble tumor necrosis factor receptors in cerebrospinal fluid of children with bacterial meningitis. J Infect Dis 173:1498–1502
Krueger M, Bechmann I (2010) CNS pericytes: concepts, misconceptions, and a way out. Glia 58:1–10
Krumbholz M, Theil D, Derfuss T et al (2005) BAFF is produced by astrocytes and up-regulated in multiple sclerosis lesions and primary central nervous system lymphoma. J Exp Med 201:195–200
Kurzwelly D, Glas M, Roth P et al (2010) Primary CNS lymphoma in the elderly: temozolomide therapy and MGMT status. J Neurooncol 97:389–392
Larocca LM, Capello D, Rinelli A et al (1998) The molecular and phenotypic profile of primary central nervous system lymphoma identifies distinct categories of the disease and is consistent with histogenetic derivation from germinal center-related B cells. Blood 92:1011–1019
Lenz G, Wright G, Dave SS et al (2008) Stromal gene signatures in large-B-cell lymphomas. N Engl J Med 359:2313–2323
Lo Coco F, Ye BH, Lista F et al (1994) Rearrangements of the BCL6 gene in diffuse large cell non-Hodgkin’s lymphoma. Blood 83:1757–1759
Mastroianni CM, Paoletti F, Lichtner M, D’Agostino C, Vullo V, Delia S (1997) Cerebrospinal fluid cytokines in patients with tuberculous meningitis. Clin Immunol Immunopathol 84:171–176
Mathieson BJ, Campbell PS, Potter M, Asofsky R (1978) Expression of Ly 1, Ly 2, Thy 1, and TL differentiation antigens on mouse T-cell tumors. J Exp Med 147:1267–1279
Matsumoto T, Imagama S, Hirano K et al (2012) CD44 expression in astrocytes and microglia is associated with ALS progression in a mouse model. Neurosci Lett 520:115–120
McHeyzer-Williams MG, McLean MJ, Nossal GJV, Lalor PA (1992) The dynamics of T cell-dependent B cell responses in vivo. Immunol Cell Biol 70:119–127
Mineo JF, Scheffer A, Karkoutly C et al (2008) Using human CD20-transfected murine lymphomatous B cells to evaluate the efficacy of intravitreal and intracerebral rituximab injections in mice. Invest Ophthalmol Vis Sci 49:4738–4745
Montesinos-Rongen M, Akasaka T, Zühlke-Jenisch R et al (2003) Molecular characterization of BCL6 breakpoints in primary diffuse large B-cell lymphomas of the central nervous system identifies GAPD as novel translocation partner. Brain Pathol 13:534–538
Montesinos-Rongen M, Brunn A, Bentink S et al (2008) Gene expression profiling suggests primary central nervous system lymphomas to be derived from a late germinal center B cell. Leukemia 22:400–405
Montesinos-Rongen M, Godlewska E, Brunn A, Wiestler OD, Siebert R, Deckert M (2011) Activating L265P mutations of the MYD88 gene are common in primary central nervous system lymphoma. Acta Neuropathol 122:791–792
Montesinos-Rongen M, Hans VH, Eis-Hübinger AM et al (2001) Human herpes virus-8 is not associated with primary central nervous system lymphoma in HIV-negative patients. Acta Neuropathol 102:489–495
Montesinos-Rongen M, Küppers R, Schlüter D et al (1999) Primary central nervous system lymphomas are derived from germinal-center B cells and show a preferential usage of the V4-34 gene segment. Am J Pathol 155:2077–2086
Montesinos-Rongen M, Sanchez-Ruiz M, Brunn A et al (2013) Mechanisms of intracerebral lymphoma growth delineated in a syngeneic mouse model of central nervous system lymphoma. J Neuropathol Exp Neurol 72:325–336
Montesinos-Rongen M, Schäfer E, Siebert R, Deckert M (2012) Genes regulating the B cell receptor pathway are recurrently mutated in primary central nervous system lymphoma. Acta Neuropathol 124:905–906
Montesinos-Rongen M, Schmitz R, Brunn A et al (2010) Mutations of CARD11 but not TNFAIP3 may activate the NF-kappaB pathway in primary CNS lymphoma. Acta Neuropathol 120:529–535
Montesinos-Rongen M, Schmitz R, Courts C et al (2005) Absence of immunoglobulin class switch in primary lymphomas of the central nervous system. Am J Pathol 166:1773–1779
Montesinos-Rongen M, Siebert R, Deckert M (2009) Primary lymphoma of the central nervous system: just DLBCL or not? Blood 113:7–10
Montesinos-Rongen M, Van Roost D, Schaller C, Wiestler OD, Deckert M (2004) Primary diffuse large B-cell lymphomas of the central nervous system are targeted by aberrant somatic hypermutation. Blood 103:1869–1875
Montesinos-Rongen M, Zühlke-Jenisch R, Gesk S et al (2002) Interphase cytogenetic analysis of lymphoma-associated chromosomal breakpoints in primary diffuse large B-cell lymphomas of the central nervous system. J Neuropathol Exp Neurol 61:926–933
Morse HC 3rd, Anver MR, Fredrickson TN et al (2002) Bethesda proposals for classification of lymphoid neoplasms in mice. Blood 100:246–258
Muldoon LL, Lewin SJ, Dosa E et al (2011) Imaging and therapy with rituximab anti-CD20 immunotherapy in an animal model of central nervous system lymphoma. Clin Cancer Res 17:2207–2215
Ngo VN, Young RM, Schmitz R et al (2011) Oncogenically active MYD88 mutations in human lymphoma. Nature 470:115–119
O’Connor PM, Wassermann K, Sarang M, Magrath I, Bohr VA, Kohn KW (1991) Relationship between DNA cross-links, cell cycle, and apoptosis in Burkitt’s lymphoma cell lines differing in sensitivity to nitrogen mustard. Cancer Res 51:6550–6557
Ochiai K, Maienschein-Cline M, Simonetti G et al (2013) Transcriptional regulation of germinal center B and plasma cell fates by dynamical control of IRF4. Immunity 38:918–929
Paulus W, Jellinger K (1993) Comparison of integrin adhesion molecules expressed by primary brain lymphomas and nodal lymphomas. Acta Neuropathol 86:360–364
Pels H, Montesinos-Rongen M, Schaller C et al (2005) VH gene analysis of primary CNS lymphomas. J Neurol Sci 228:143–147
Pesic M, Bartholomäus I, Kyratsous NI, Heissmeyer V, Wekerle H, Kawakami N (2013) 2-photon imaging of phagocyte-mediated T cell activation in the CNS. J Clin Invest 123:1192–1201
Ponzoni M, Berger F, Chassagne-Clement C et al (2007) Reactive perivascular T-cell infiltrate predicts survival in primary central nervous system B-cell lymphomas. Br J Haematol 138:316–323
Ramkumar HL, de Shen F, Tuo J et al (2012) IL-10-1082 SNP and IL-10 in primary CNS and vitreoretinal lymphomas. Graefes Arch Clin Exp Ophthalmol 250:1541–1548
Richter J, Ammerpohl O, Martin-Subero JI et al (2009) Array-based DNA methylation profiling of primary lymphomas of the central nervous system. BMC Cancer 9:455–462
Rickert CH, Dockhorn-Dworniczak B, Simon R, Paulus W (1999) Chromosomal imbalances in primary lymphomas of the central nervous system. Am J Pathol 155:1445–1451
Riemersma SA, Oudejans JJ, Vonk MJ et al (2005) High numbers of tumour-infiltrating activated cytotoxic T lymphocytes, and frequent loss of HLA class I and II expression, are features of aggressive B cell lymphomas of the brain and testis. J Pathol 206:328–336
Rimsza LM, Roberts RA, Miller TP et al (2004) Loss of MHC class II gene and protein expression in diffuse large B-cell lymphoma is related to decreased tumor immunosurveillance and poor patient survival regardless of other prognostic factors: a follow-up study from the Leukemia and Lymphoma Molecular Profiling Project. Blood 103:4251–4258
Robertus JL, Harms G, Blokzijl T et al (2009) Specific expression of miR-17-5p and miR-127 in testicular and central nervous system diffuse large B-cell lymphoma. Mod Pathol 22:547–555
Roers A, Siewe L, Strittmatter E et al (2004) T cell-specific inactivation of the interleukin 10 gene in mice results in enhanced T cell responses but normal innate responses to lipopolysaccharide or skin irritation. J Exp Med 200:1289–1297
Rössler K, Neuchrist C, Kitz K, Scheiner O, Kraft D, Lassmann H (1992) Expression of leucocyte adhesion molecules at the human blood-brain barrier (BBB). J Neurosci Res 31:365–374
Roychowdhury S, Peng R, Baiocchi RA et al (2003) Experimental treatment of Epstein–Barr virus-associated primary central nervous system lymphoma. Cancer Res 63:965–971
Rubenstein JL, Fridlyand J, Shen A et al (2006) Gene expression and angiotropism in primary CNS lymphoma. Blood 107:3716–3723
Rubenstein JL, Wong VS, Kadoch C et al (2013) CXCL13 plus interleukin 10 is highly specific for the diagnosis of CNS lymphoma. Blood 121:4740–4748
Sagardoy A, Martinez-Ferrandis JI, Roa S et al (2013) Downregulation of FOXP1 is required during germinal center B-cell function. Blood 121:4311–4320
Schlüter D, Löhler J, Deckert M, Hof H, Schwendemann G (1991) Toxoplasma encephalitis of immunocompetent and nude mice: immunohistochemical characterisation of toxoplasma antigen, infiltrates and major histocompatibility complex gene products. J Neuroimmunol 31:185–198
Schlüter D, Oprisiu SB, Chahoud S et al (1995) Systemic immunization induces protective CD4+ and CD8+ T cell-mediated immune responses in murine Listeria monocytogenes meningoencephalitis. Eur J Immunol 25:2384–2391
Schwindt H, Akasaka T, Zühlke-Jenisch R et al (2006) Chromosomal translocations fusing the BCL6 gene to different partner loci are recurrent in primary central nervous system lymphoma and may be associated with aberrant somatic hypermutation or defective class switch recombination. J Neuropathol Exp Neurol 65:776–782
Schwindt H, Vater I, Kreuz M et al (2009) Chromosomal imbalances and partial uniparental disomies in primary central nervous system lymphoma. Leukemia 23:1875–1884
Shapiro-Shelef M, Lin KI, McHeyzer-Williams LJ, Liao J, McHeyzer-Williams MG, Calame K (2003) Blimp-1 is required for the formation of immunoglobulin secreting plasma cells and pre-plasma memory B cells. Immunity 19:607–620
Shin T, Ahn M, Kim H et al (2005) Temporal expression of osteopontin and CD44 in rat brains with experimental cryolesions. Brain Res 1041:95–101
Soussain C, Muldoon LL, Varallyay C, Jahnke K, DePaula L, Neuwelt EA (2007) Characterization and magnetic resonance imaging of a rat model of human B-cell central nervous system lymphoma. Clin Cancer Res 13:2504–2511
Stenzel W, Dahm J, Sanchez-Ruiz M et al (2005) Regulation of the inflammatory response to Staphylococcus aureus-induced brain abscess by interleukin-10. J Neuropathol Exp Neurol 64:1046–1057
Thompsett AR, Ellison DW, Stevenson FK, Zhu D (1999) V(H) gene sequences from primary central nervous system lymphomas indicate derivation from highly mutated germinal center B cells with ongoing mutational activity. Blood 94:1738–1746
Torre D, Zeroli C, Martegani R, Speranza F (1996) Levels of interleukin-10 and tumor necrosis factor alpha in patients with bacterial meningitis. Clin Infect Dis 22:883–885
van Furth AM, Seijmonsbergen EM, Langermans JA, Groeneveld PH, de Bel CE, van Furth R (1995) High levels of interleukin 10 and tumor necrosis factor alpha in cerebrospinal fluid during the onset of bacterial meningitis. Clin Infect Dis 21:220–222
Weber T, Weber RG, Kaulich K et al (2000) Characteristic chromosomal imbalances in primary central nervous system lymphomas of the diffuse large B-cell type. Brain Pathol 10:73–84
Ye BH, Lista F, Lo Coco F et al (1993) Alterations of a zinc finger-encoding gene, BCL-6, in diffuse large-cell lymphoma. Science 262:747–750
Yuan J, Gu K, He J, Sharma S (2013) Preferential up-regulation of osteopontin in primary central nervous system lymphoma does not correlate with putative receptor CD44v6 or CD44H expression. Hum Pathol 44:606–611
Acknowledgments
The authors’ work has been supported by the Deutsche Krebshilfe (grant no.: 109471), the Wilhelm-Sander-Stiftung (2011.092.1), and the German Ministry for Education and Science(BMBF) through ICGC MMML-Seq (01KU1002A-J). We thank Katherine Dege for critical reading of the manuscript. We also acknowledge the work of all authors who could not be appropriately cited and are greatly indebted for the continuous support of the many colleagues who have provided material or clinical information over many years. This is a strong obligation for us to pursue our studies of PCNSL.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Deckert, M., Montesinos-Rongen, M., Brunn, A. et al. Systems biology of primary CNS lymphoma: from genetic aberrations to modeling in mice. Acta Neuropathol 127, 175–188 (2014). https://doi.org/10.1007/s00401-013-1202-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-013-1202-x