Abstract
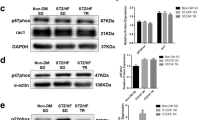
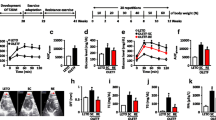
Obesity and diabetes are associated with higher cardiac vulnerability to ischemia–reperfusion (IR). The cardioprotective effect of regular exercise has been attributed to β3-adrenergic receptor (β3AR) stimulation and increased endothelial nitric oxide synthase (eNOS) activation. Here, we evaluated the role of the β3AR–eNOS pathway and NOS isoforms in exercise-induced cardioprotection of C57Bl6 mice fed with high fat and sucrose diet (HFS) for 12 weeks and subjected or not to exercise training during the last 4 weeks (HFS-Ex). HFS animals were more sensitive to in vivo and ex vivo IR injuries than control (normal diet) and HFS-Ex mice. Cardioprotection in HFS-Ex mice was not associated with increased myocardial eNOS activation and NO metabolites storage, possibly due to the β3AR–eNOS pathway functional loss in their heart. Indeed, a selective β3AR agonist (BRL37344) increased eNOS activation and had a protective effect against IR in control, but not in HFS hearts. Moreover, iNOS expression, nitro-oxidative stress (protein s-nitrosylation and nitrotyrosination) and ROS production during early reperfusion were increased in HFS, but not in control mice. Exercise normalized iNOS level and reduced protein s-nitrosylation, nitrotyrosination and ROS production in HFS-Ex hearts during early reperfusion. The iNOS inhibitor 1400 W reduced in vivo infarct size in HFS mice to control levels, supporting the potential role of iNOS normalization in the cardioprotective effects of exercise training in HFS-Ex mice. Although the β3AR–eNOS pathway is defective in the heart of HFS mice, regular exercise can protect their heart against IR by reducing iNOS expression and nitro-oxidative stress.




Similar content being viewed by others
References
Alegria JR, Miller TD, Gibbons RJ, Yi QL, Yusuf S, Collaborative Organization of RheothRx Evaluation (CORE) Trial Investigators (2007) Infarct size, ejection fraction, and mortality in diabetic patients with acute myocardial infarction treated with thrombolytic therapy. Am Heart J 154:743–750. doi:10.1016/j.ahj.2007.06.020
Aragón JP, Condit ME, Bhushan S, Predmore BL, Patel SS, Grinsfelder DB, Gundewar S, Jha S, Calvert JW, Barouch LA, Lavu M, Wright HM, Lefer DJ (2011) β3-adrenoreceptor stimulation ameliorates myocardial ischemia-reperfusion injury via endothelial nitric oxide synthase and neuronal nitric oxide synthase activation. J Am Coll Cardiol 58:2683–2691. doi:10.1016/j.jacc.2011.09.033
Barba I, Chavarria L, Ruiz-Meana M, Mirabet M, Agulló E, Garcia-Dorado D (2009) Effect of intracellular lipid droplets on cytosolic Ca2+ and cell death during ischaemia-reperfusion injury in cardiomyocytes. J Physiol 587:1331–1341. doi:10.1113/jphysiol.2008.163311
Belaidi E, Thomas A, Bourdier G, Moulin S, Lemarié E, Levy P, Pépin JL, Korichneva I, Godin-Ribuot D, Arnaud C (2016) Endoplasmic reticulum stress as a novel inducer of hypoxia inducible factor-1 activity: its role in the susceptibility to myocardial ischemia-reperfusion induced by chronic intermittent hypoxia. Int J Cardiol 210:45–53. doi:10.1016/j.ijcard.2016.02.096
Birenbaum A, Tesse A, Loyer X, Michelet P, Andriantsitohaina R, Heymes C, Riou B, Amour J (2008) Involvement of β3-adrenoceptor in altered beta-adrenergic response in senescent heart: role of nitric oxide synthase 1-derived nitric oxide. Anesthesiology 109:1045–1053. doi:10.1097/ALN.0b013e31818d7e5a
Bolli R (2007) Preconditioning: a paradigm shift in the biology of myocardial ischemia. Am J Physiol Heart Circ Physiol 292:H19–H27. doi:10.1152/ajpheart.00712.2006
Calvert JW, Condit ME, Aragón JP, Nicholson CK, Moody BF, Hood RL, Sindler AL, Gundewar S, Seals DR, Barouch LA, Lefer DJ (2011) Exercise protects against myocardial ischemia-reperfusion injury via stimulation of β3-adrenergic receptors and increased nitric oxide signaling: role of nitrite and nitrosothiols. Circ Res 108:1448–1458. doi:10.1161/CIRCRESAHA.111.241117
Farah C, Kleindienst A, Bolea G, Meyer G, Gayrard S, Geny B, Obert P, Cazorla O, Tanguy S, Reboul C (2013) Exercise-induced cardioprotection: a role for eNOS uncoupling and NO metabolites. Basic Res Cardiol 108:389. doi:10.1007/s00395-013-0389-2
Ferdinandy P, Hausenloy DJ, Heusch G, Baxter GF, Schulz R (2014) Interaction of risk factors, comorbidities, and comedications with ischemia/reperfusion injury and cardioprotection by preconditioning, postconditioning, and remote conditioning. Pharmacol Rev 66:1142–1174. doi:10.1124/pr.113.008300
Fève B, Elhadri K, Quignard-Boulangé A, Pairault J (1994) Transcriptional down-regulation by insulin of the β3-adrenergic receptor expression in 3T3-F442A adipocytes: a mechanism for repressing the cAMP signaling pathway. Proc Natl Acad Sci USA 91:5677–5681
French JP, Hamilton KL, Quindry JC, Lee Y, Upchurch PA, Powers SK (2008) Exercise-induced protection against myocardial apoptosis and necrosis: MnSOD, calcium-handling proteins, and calpain. FASEB J Off Publ Fed Am Soc Exp Biol 22:2862–2871. doi:10.1096/fj.07-102541
García-Prieto J, García-Ruiz JM, Sanz-Rosa D, Pun A, García-Alvarez A, Davidson SM, Fernández-Friera L, Nuno-Ayala M, Fernández-Jiménez R, Bernal JA, Izquierdo-Garcia JL, Jimenez-Borreguero J, Pizarro G, Ruiz-Cabello J, Macaya C, Fuster V, Yellon DM, Ibanez B (2014) β3 adrenergic receptor selective stimulation during ischemia/reperfusion improves cardiac function in translational models through inhibition of mPTP opening in cardiomyocytes. Basic Res Cardiol 109:422. doi:10.1007/s00395-014-0422-0
Gödecke A, Molojavyi A, Heger J, Flögel U, Ding Z, Jacoby C, Schrader J (2003) Myoglobin protects the heart from inducible nitric-oxide synthase (iNOS)-mediated nitrosative stress. J Biol Chem 278:21761–21766. doi:10.1074/jbc.M302573200
Guo Y, Sanganalmath SK, Wu W, Zhu X, Huang Y, Tan W, Ildstad ST, Li Q, Bolli R (2012) Identification of inducible nitric oxide synthase in peripheral blood cells as a mediator of myocardial ischemia/reperfusion injury. Basic Res Cardiol 107:253. doi:10.1007/s00395-012-0253-9
Guo Y, Stein AB, Wu WJ, Zhu X, Tan W, Li Q, Bolli R (2005) Late preconditioning induced by NO donors, adenosine A1 receptor agonists, and δ1-opioid receptor agonists is mediated by iNOS. Am J Physiol Heart Circ Physiol 289:H2251–H2257. doi:10.1152/ajpheart.00341.2005
Hadri KE, Charon C, Pairault J, Hauguel-De Mouzon S, Quignard-Boulangé A, Fève B (1997) Down-regulation of β3-adrenergic receptor expression in rat adipose tissue during the fasted/fed transition: evidence for a role of insulin. Biochem J 323(Pt 2):359–364
von Harsdorf R, Li PF, Dietz R (1999) Signaling pathways in reactive oxygen species-induced cardiomyocyte apoptosis. Circulation 99:2934–2941
Heusch G (2011) β3-adrenoceptor activation just says NO to myocardial reperfusion injury. J Am Coll Cardiol 58:2692–2694. doi:10.1016/j.jacc.2011.09.034
Hu A, Jiao X, Gao E, Koch WJ, Sharifi-Azad S, Grunwald Z, Ma XL, Sun JZ (2006) Chronic beta-adrenergic receptor stimulation induces cardiac apoptosis and aggravates myocardial ischemia/reperfusion injury by provoking inducible nitric-oxide synthase-mediated nitrative stress. J Pharmacol Exp Ther 318:469–475. doi:10.1124/jpet.106.102160
Huang JV, Lu L, Ye S, Bergman BC, Sparagna GC, Sarraf M, Reusch JEB, Greyson CR, Schwartz GG (2013) Impaired contractile recovery after low-flow myocardial ischemia in a porcine model of metabolic syndrome. Am J Physiol Heart Circ Physiol 304:H861–H873. doi:10.1152/ajpheart.00535.2012
Idigo WO, Reilly S, Zhang MH, Zhang YH, Jayaram R, Carnicer R, Crabtree MJ, Balligand JL, Casadei B (2012) Regulation of endothelial nitric-oxide synthase (NOS) S-glutathionylation by neuronal NOS: evidence of a functional interaction between myocardial constitutive NOS isoforms. J Biol Chem 287:43665–43673. doi:10.1074/jbc.M112.412031
Larson JE, Rainer PP, Watts VL, Yang R, Miller KL, Phan A, Barouch LA (2012) Dependence of β3-adrenergic signaling on the adipokine leptin in cardiac myocytes. Int J Obes 36:876–879. doi:10.1038/ijo.2011.137
Lei P, Baysa A, Nebb HI, Valen G, Skomedal T, Osnes JB, Yang Z, Haugen F (2013) Activation of Liver X receptors in the heart leads to accumulation of intracellular lipids and attenuation of ischemia-reperfusion injury. Basic Res Cardiol 108:323. doi:10.1007/s00395-012-0323-z
Lekli I, Szabo G, Juhasz B, Das S, Das M, Varga E, Szendrei L, Gesztelyi R, Varadi J, Bak I, Das DK, Tosaki A (2008) Protective mechanisms of resveratrol against ischemia-reperfusion-induced damage in hearts obtained from Zucker obese rats: the role of GLUT-4 and endothelin. Am J Physiol Heart Circ Physiol 294:H859–H866. doi:10.1152/ajpheart.01048.2007
Levantesi G, Macchia A, Marfisi R, Franzosi MG, Maggioni AP, Nicolosi GL, Schweiger C, Tavazzi L, Tognoni G, Valagussa F, Marchioli R, GISSI-Prevenzione Investigators (2005) Metabolic syndrome and risk of cardiovascular events after myocardial infarction. J Am Coll Cardiol 46:277–283. doi:10.1016/j.jacc.2005.03.062
Li Q, Guo Y, Ou Q, Cui C, Wu WJ, Tan W, Zhu X, Lanceta LB, Sanganalmath SK, Dawn B, Shinmura K, Rokosh GD, Wang S, Bolli R (2009) Gene transfer of inducible nitric oxide synthase affords cardioprotection by upregulating heme oxygenase-1 via a nuclear factor-{kappa}B-dependent pathway. Circulation 120:1222–1230. doi:10.1161/CIRCULATIONAHA.108.778688
Lund J, Hafstad AD, Boardman NT, Rossvoll L, Rolim NP, Ahmed MS, Florholmen G, Attramadal H, Wisløff U, Larsen TS, Aasum E (2015) Exercise training promotes cardioprotection through oxygen-sparing action in high fat-fed mice. Am J Physiol Heart Circ Physiol 308:H823–H829. doi:10.1152/ajpheart.00734.2014
Marso SP, Miller T, Rutherford BD, Gibbons RJ, Qureshi M, Kalynych A, Turco M, Schultheiss HP, Mehran R, Krucoff MW, Lansky AJ, Stone GW (2007) Comparison of myocardial reperfusion in patients undergoing percutaneous coronary intervention in ST-segment elevation acute myocardial infarction with versus without diabetes mellitus (from the EMERALD Trial). Am J Cardiol 100:206–210. doi:10.1016/j.amjcard.2007.02.080
Meyer G, André L, Kleindienst A, Singh F, Tanguy S, Richard S, Obert P, Boucher F, Jover B, Cazorla O, Reboul C (2015) Carbon monoxide increases inducible NOS expression that mediates CO-induced myocardial damage during ischemia-reperfusion. Am J Physiol Heart Circ Physiol 308:H759–H767. doi:10.1152/ajpheart.00702.2014
Meyer G, André L, Tanguy S, Boissiere J, Farah C, Lopez-Lauri F, Gayrard S, Richard S, Boucher F, Cazorla O, Obert P, Reboul C (2010) Simulated urban carbon monoxide air pollution exacerbates rat heart ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 298:H1445–H1453. doi:10.1152/ajpheart.01194.2009
Miki T, Itoh T, Sunaga D, Miura T (2012) Effects of diabetes on myocardial infarct size and cardioprotection by preconditioning and postconditioning. Cardiovasc Diabetol 11:67. doi:10.1186/1475-2840-11-67
Mozaffari MS, Schaffer SW (2008) Myocardial ischemic-reperfusion injury in a rat model of metabolic syndrome. Obes Silver Spring Md 16:2253–2258. doi:10.1038/oby.2008.356
Nduhirabandi F, Du Toit EF, Blackhurst D, Marais D, Lochner A (2011) Chronic melatonin consumption prevents obesity-related metabolic abnormalities and protects the heart against myocardial ischemia and reperfusion injury in a prediabetic model of diet-induced obesity. J Pineal Res 50:171–182. doi:10.1111/j.1600-079X.2010.00826.x
Nistri S, Boccalini G, Bencini A, Becatti M, Valtancoli B, Conti L, Lucarini L, Bani D (2015) A new low molecular weight, MnII-containing scavenger of superoxide anion protects cardiac muscle cells from hypoxia/reoxygenation injury. Free Radic Res 49:67–77. doi:10.3109/10715762.2014.979168
Niu X, Watts VL, Cingolani OH, Sivakumaran V, Leyton-Mange JS, Ellis CL, Miller KL, Vandegaer K, Bedja D, Gabrielson KL, Paolocci N, Kass DA, Barouch LA (2012) Cardioprotective effect of β3 adrenergic receptor agonism: role of neuronal nitric oxide synthase. J Am Coll Cardiol 59:1979–1987. doi:10.1016/j.jacc.2011.12.046
Osipov RM, Bianchi C, Feng J, Clements RT, Liu Y, Robich MP, Glazer HP, Sodha NR, Sellke FW (2009) Effect of hypercholesterolemia on myocardial necrosis and apoptosis in the setting of ischemia-reperfusion. Circulation 120:S22–S30. doi:10.1161/CIRCULATIONAHA.108.842724
Pons S, Martin V, Portal L, Zini R, Morin D, Berdeaux A, Ghaleh B (2013) Regular treadmill exercise restores cardioprotective signaling pathways in obese mice independently from improvement in associated co-morbidities. J Mol Cell Cardiol 54:82–89. doi:10.1016/j.yjmcc.2012.11.010
Powers SK, Smuder AJ, Kavazis AN, Quindry JC (2014) Mechanisms of exercise-induced cardioprotection. Physiol Bethesda Md 29:27–38. doi:10.1152/physiol.00030.2013
Przyklenk K (2011) Efficacy of cardioprotective “conditioning” strategies in aging and diabetic cohorts: the co-morbidity conundrum. Drugs Aging 28:331–343. doi:10.2165/11587190-000000000-00000
Roof SR, Ho HT, Little SC, Ostler JE, Brundage EA, Periasamy M, Villamena FA, Györke S, Biesiadecki BJ, Heymes C, Houser SR, Davis JP, Ziolo MT (2015) Obligatory role of neuronal nitric oxide synthase in the heart’s antioxidant adaptation with exercise. J Mol Cell Cardiol 81:54–61. doi:10.1016/j.yjmcc.2015.01.003
Soliman H, Craig GP, Nagareddy P, Yuen VG, Lin G, Kumar U, McNeill JH, Macleod KM (2008) Role of inducible nitric oxide synthase in induction of RhoA expression in hearts from diabetic rats. Cardiovasc Res 79:322–330. doi:10.1093/cvr/cvn095
Song D, Kuo KH, Yao R, Hutchings SR, Pang CCY (2008) Inducible nitric oxide synthase depresses cardiac contractile function in Zucker diabetic fatty rats. Eur J Pharmacol 579:253–259. doi:10.1016/j.ejphar.2007.09.043
Tavernier G, Toumaniantz G, Erfanian M, Heymann MF, Laurent K, Langin D, Gauthier C (2003) β3-Adrenergic stimulation produces a decrease of cardiac contractility ex vivo in mice overexpressing the human β3-adrenergic receptor. Cardiovasc Res 59:288–296
Trappanese DM, Liu Y, McCormick RC, Cannavo A, Nanayakkara G, Baskharoun MM, Jarrett H, Woitek FJ, Tillson DM, Dillon AR, Recchia FA, Balligand JL, Houser SR, Koch WJ, Dell’Italia LJ, Tsai EJ (2015) Chronic β1-adrenergic blockade enhances myocardial β3-adrenergic coupling with nitric oxide-cGMP signaling in a canine model of chronic volume overload: new insight into mechanisms of cardiac benefit with selective β1-blocker therapy. Basic Res Cardiol 110:456. doi:10.1007/s00395-014-0456-3
Wang T, Mao X, Li H, Qiao S, Xu A, Wang J, Lei S, Liu Z, Ng KFJ, Wong GT, Vanhoutte PM, Irwin MG, Xia Z (2013) N-Acetylcysteine and allopurinol up-regulated the Jak/STAT3 and PI3K/Akt pathways via adiponectin and attenuated myocardial postischemic injury in diabetes. Free Radic Biol Med 63:291–303. doi:10.1016/j.freeradbiomed.2013.05.043
Wang XL, Liu HR, Tao L, Liang F, Yan L, Zhao RR, Lopez BL, Christopher TA, Ma XL (2007) Role of iNOS-derived reactive nitrogen species and resultant nitrative stress in leukocytes-induced cardiomyocyte apoptosis after myocardial ischemia/reperfusion. Apoptosis Int J Program Cell Death 12:1209–1217. doi:10.1007/s10495-007-0055-y
Wang Y, Guo Y, Zhang SX, Wu WJ, Wang J, Bao W, Bolli R (2002) Ischemic preconditioning upregulates inducible nitric oxide synthase in cardiac myocyte. J Mol Cell Cardiol 34:5–15. doi:10.1006/jmcc.2001.1482
Weidinger A, Müllebner A, Paier-Pourani J, Banerjee A, Miller I, Lauterböck L, Duvigneau JC, Skulachev VP, Redl H, Kozlov AV (2015) Vicious inducible nitric oxide synthase-mitochondrial reactive oxygen species cycle accelerates inflammatory response and causes liver injury in rats. Antioxid Redox Signal 22:572–586. doi:10.1089/ars.2014.5996
Yi W, Sun Y, Gao E, Wei X, Lau WB, Zheng Q, Wang Y, Yuan Y, Wang X, Tao L, Li R, Koch W, Ma XL (2011) Reduced cardioprotective action of adiponectin in high-fat diet-induced type II diabetic mice and its underlying mechanisms. Antioxid Redox Signal 15:1779–1788. doi:10.1089/ars.2010.3722
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by the “Société Francophone du Diabète”, the “région Provence Alpes Côtes d’Azur”, the “Structure Fédérative de Recherche” TERSYS. SB was granted by the” Groupe de reflexion sur la Recherche Cardiovasculaire (GRRC)” for its experimental work conducted in Grenoble.
Conflict of interest
No potential conflicts of interest relevant to this article were reported.
Additional information
A. Kleindienst and S. Battault contributed equally to this work.
O. Cazorla, C. Reboul are senoir co-authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kleindienst, A., Battault, S., Belaidi, E. et al. Exercise does not activate the β3 adrenergic receptor–eNOS pathway, but reduces inducible NOS expression to protect the heart of obese diabetic mice. Basic Res Cardiol 111, 40 (2016). https://doi.org/10.1007/s00395-016-0559-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-016-0559-0