Abstract
Purpose
Mushrooms are reported to have a variety of health-promoting activities. However, little information is available on the effects of intake of polysaccharides from Pleurotus eryngii on obesity. In this study, we investigated the effects of P. eryngii polysaccharides on obesity and gut microbiota in mice fed a high-fat diet.
Methods
Soluble polysaccharides were extracted from P. eryngii using hot water. C57BL/6J mice were fed a standard diet (ST), a high-fat diet (HF), or HF with 1% or 5% P. eryngii polysaccharide fraction (LP or HP) for 16 weeks. Adipose tissues were weighed and blood parameters were measured. Expression of genes involved in fatty acid and cholesterol metabolism was assessed by real-time quantitative PCR. The gut microbiota composition was analysed by 16S rRNA gene sequencing.
Results
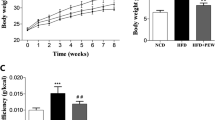
Body weight gain and mesenteric fat tissue were lower in the HP group than in the HF group. In the HP group, serum total cholesterol and LDL cholesterol levels decreased, and lipid and total bile acids in faeces increased. Mice in the HP group showed increased expression of the LDLR gene in the liver and GPR43 in fat. The relative abundance of Firmicutes was significantly higher in the HF and HP groups than in the ST group. The abundance of some short-chain fatty acid-producing gut bacteria was altered by P. eryngii polysaccharides.
Conclusions
These results provide the first evidence that P. eryngii polysaccharides have anti-obesity and LDL cholesterol-lowering effects in obese mice through increased excretion of bile acids and lipids and altered microbiota.












Similar content being viewed by others
References
NCD Risk Factor Collaboration (NCD-RisC) (2016) Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet 387:1377–1396. https://doi.org/10.1016/S0140-6736(16)30054-X
Apovian CM, Aronne LJ, Bessesen DH, McDonnell ME, Murad MH, Pagotto U, Ryan DH, Still CD (2015) Endocrine society. pharmacological management of obesity: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 100:342–362. https://doi.org/10.1210/jc.2014-3415
Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI (2005) Obesity alters gut microbial ecology. Proc Natl Acad Sci USA 102:11070–11075. https://doi.org/10.1073/pnas.0504978102
Ley RE, Turnbaugh PJ, Klein S, Gordon JI (2006) Microbial ecology: human gut microbes associated with obesity. Nature 444:1022–1023. https://doi.org/10.1038/4441022a
Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI (2006) An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444:1027–1031. https://doi.org/10.1038/nature05414
Bäckhed F, Manchester JK, Semenkovich CF, Gordon JI (2007) Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci USA 104:979–984. https://doi.org/10.1073/pnas.0605374104
Rabot S, Membrez M, Bruneau A, Gérard P, Harach T, Moser M, Raymond F, Mansourian R, Chou CJ (2010) Germ-free C57BL/6J mice are resistant to high-fat-diet-induced insulin resistance and have altered cholesterol metabolism. FASEB J 24:4948–4959. https://doi.org/10.1096/fj.10-164921
Holscher HD (2017) Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 8:172–184. https://doi.org/10.1080/19490976.2017.1290756
Sonnenburg JL, Bäckhed F (2016) Diet-microbiota interactions as moderators of human metabolism. Nature 535:56–64. https://doi.org/10.1038/nature18846
Nicolucci AC, Hume MP, Martínez I, Mayengbam S, Walter J, Reimer RA (2017) Prebiotics reduce body fat and alter intestinal microbiota in children who are overweight or with obesity. Gastroenterology 153:711–722. https://doi.org/10.1053/j.gastro.2017.05.055
Jiang T, Gao X, Wu C, Tian F, Lei Q, Bi J, Xie B, Wang HY, Chen S, Wang X (2016) Apple-derived pectin modulates gut microbiota, improves gut barrier function, and attenuates metabolic endotoxemia in rats with diet-induced obesity. Nutrients 8:126. https://doi.org/10.3390/nu8030126
Li X, Guo J, Ji K, Zhang P (2016) Bamboo shoot fiber prevents obesity in mice by modulating the gut microbiota. Sci Rep 6:32953. https://doi.org/10.1038/srep32953
Yang Z, Xu J, Fu Q, Fu X, Shu T, Bi Y, Song B (2013) Antitumor activity of a polysaccharide from Pleurotus eryngii on mice bearing renal cancer. Carbohydr Polym 95:615–620. https://doi.org/10.1016/j.carbpol.2013.03.024
Zhang C, Li S, Zhang J, Hu C, Che G, Zhou M, Jia L (2016) Antioxidant and hepatoprotective activities of intracellular polysaccharide from Pleurotus eryngii SI-04. Int J Biol Macromol 91:568–577. https://doi.org/10.1016/j.ijbiomac.2016.05.104
Xu D, Wang H, Zheng W, Gao Y, Wang M, Zhang Y, Gao Q (2016) Charaterization and immunomodulatory activities of polysaccharide isolated from Pleurotus eryngii. Int J Biol Macromol 92:30–36. https://doi.org/10.1016/j.ijbiomac.2016.07.016
Ma G, Kimatu BM, Zhao L, Yang W, Pei F, Hu Q (2017) In vivo fermentation of a Pleurotus eryngii polysaccharide and its effects on fecal microbiota composition and immune response. Food Funct 8(5):1810–1821. https://doi.org/10.1039/c7fo00341b
Mosikanon K, Arthan D, Kettawan A, Tungtrongchitr R, Prangthip P (2017) Yeast β-glucan modulates inflammation and waist circumference in overweight and obese subjects. J Diet 14(2):173–185. https://doi.org/10.1080/19390211.2016.1207005
Miyamoto J, Watanabe K, Taira S, Kasubuchi M, Li X, Irie J, Itoh H, Kimura I (2018) Barley β-glucan improves metabolic condition via short-chain fatty acids produced by gut microbial fermentation in high fat diet fed mice. PLoS ONE 13(4):5e0196579. https://doi.org/10.1371/journal.pone.0196579
Dubios M, Gilles KA, Rebers PA, Smith F (1956) Calorimetric dubois method for determination of sugar and related substances. Anal Chem 28:350–356. https://doi.org/10.1021/ac60111a017
Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18:499–502 (PMID: 4337382)
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509 (PMID: 13428781)
Hosoyamada Y, Yamada M (2017) Effect of dietary fish oil and apple polyphenol on the concentration serum lipids and excreation of fecal bile acids in Rats. J Nutr Sci Vitaminol 63:21–27. https://doi.org/10.3177/jnsv.63.21
Takagi T, Naito Y, Inoue R, Kashiwagi S, Uchiyama K, Mizushima K, Tsuchiya S, Dohi O, Yoshida N, Kamada K, Ishikawa T, Handa O, Konishi H, Okuda K, Tsujimoto Y, Ohnogi H, Itoh Y (2019) Differences in gut microbiota associated with age, sex, and stool consistency in healthy Japanese subjects. J Gastroenterol 54:53–63. https://doi.org/10.1007/s00535-018-1488-5
Li W, Fu L, Niu B, Wu S, Wooley J (2012) Ultrafast clustering algorithms for metagenomic sequence analysis. Brief Bioinform 13(6):656–668. https://doi.org/10.1093/bib/bbs035
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7(5):335–336. https://doi.org/10.1038/nmeth.f.303
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215(3):403–410. https://doi.org/10.1016/S0022-2836(05)80360-2
Wang Q, Garrity GM, Tiedje JM, Cole JR (2007) Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73(16):5261–5267. https://doi.org/10.1128/AEM.00062-07
DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P, Andersen GL (2006) Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol 72(7):5069–5072. https://doi.org/10.1128/AEM.03006-05
Synytsya A, Míčková K, Synytsya A, Jablonský I, Spěváček J, Erban V, Kováříková E, Čopíková J (2009) Glucans from fruit bodies of cultivated mushrooms Pleurotus ostreatus and Pleurotus eryngii: structure and potential prebiotic activity. Carbohydr Polym 76:548–556. https://doi.org/10.1016/j.carbpol.2008.11.021
Synytsya A, Novak M (2014) Structural analysis of glucans. Ann Transl Med 2:17. https://doi.org/10.3978/j.issn.2305-5839.2014.02.07
Chen J, Yong Y, Xing M, Gu Y, Zhang Z, Zhang S, Lu L (2013) Characterization of polysaccharides with marked inhibitory effect on lipid accumulation in Pleurotus eryngii. Carbohydr Polym 97:604–613. https://doi.org/10.1016/j.carbpol.2013.05.028
Palanisamy M, Aldars-García L, Gil-Ramírez A, Ruiz-Rodríguez A, Marín FR, Reglero G, Soler-Rivas C (2014) Pressurized water extraction of β-glucan enriched fractions with bile acids-binding capacities obtained from edible mushrooms. Biotechnol Prog 30(2):391–400. https://doi.org/10.1002/btpr.1865
Gunness P, Gidley MJ (2010) Mechanisms underlying the cholesterol-lowering properties of soluble dietary fibre polysaccharides. Food Funct 1(2):149–155. https://doi.org/10.1039/c0fo00080a
Cnop M, Havel PJ, Utzschneider KM, Carr DB, Sinha MK, Boyko EJ, Retzlaff BM, Knopp RH, Brunzell JD, Kahn SE (2003) Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia 46:459–469. https://doi.org/10.1007/s00125-003-1074-z
Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T (2001) The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 7:941–946. https://doi.org/10.1038/90984
Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, Iwahashi H, Kuriyama H, Ouchi N, Maeda K, Nishida M, Kihara S, Sakai N, Nakajima T, Hasegawa K, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Hanafusa T, Matsuzawa Y (2000) Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol 20:1595–1599 (PMID: 10845877)
Hong YH, Nishimura Y, Hishikawa D, Tsuzuki H, Miyahara H, Gotoh C, Choi KC, Feng DD, Chen C, Lee HG, Katoh K, Roh SG, Sasaki S (2005) Acetate and propionate short chain fatty acids stimulate adipogenesis via GPCR43. Endocrinology 146:5092–5099. https://doi.org/10.1210/en.2005-0545
Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, Xavier RJ, Teixeira MM, Mackay CR (2009) Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461:1282–1286. https://doi.org/10.1210/en.2005-0545
Kimura I, Ozawa K, Inoue D, Imamura T, Kimura K, Maeda T, Terasawa K, Kashihara D, Hirano K, Tani T, Takahashi T, Miyauchi S, Shioi G, Inoue H, Tsujimoto G (2013) The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat Commun 4:1829. https://doi.org/10.1038/ncomms2852
Lu Y, Fan C, Li P, Lu Y, Chang X, Qi K (2016) Short chain fatty acids prevent high-fat-diet-induced obesity in mice by regulating G protein-coupled receptors and gut microbiota. Sci Rep 6:37589. https://doi.org/10.1038/srep37589
Brockman DA, Chen X, Gallaher DD (2013) Consumption of a high β-glucan barley flour improves glucose control and fatty liver and increases muscle acylcarnitines in the Zucker diabetic fatty rat. Eur J Nutr 52:1743–1753. https://doi.org/10.1007/s00394-012-0478-2
Li L, Guo WL, Zhang W, Xu JX, Qian M, Bai WD, Zhang YY, Rao PF, Ni L, Lv XC (2019) Grifola frondosa polysaccharides ameliorate lipid metabolic disorders and gut microbiota dysbiosis in high-fat diet fed rats. Food Funct 10(5):2560–2572. https://doi.org/10.1039/c9fo00075e
Shimizu T, Mori K, Ouchi K, Kushida M, Tsuduki T (2018) Effects of dietary intake of Japanese mushroom gut microbiota in mice. Nutrients. https://doi.org/10.3390/nu10050610
Pan YY, Zeng F, Guo WL, Li TT, Jia RB, Huang ZR, Lv XC, Zhang J, Liu B (2018) Effect of Grifola frondosa 95% ethanol extract on lipid metabolism and gut microbiota composition in high-fat diet-fed rats. Food Funct 9(12):6268–6278. https://doi.org/10.1039/c8fo01116h
Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI (2004) The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA 101:15718–15723. https://doi.org/10.1073/pnas.0407076101
Weitkunat K, Stuhlmann C, Postel A, Rumberger S, Fankhänel M, Woting A, Petzke KJ, Gohlke S, Schulz TJ, Blaut M, Klaus S, Schumann S (2017) Short-chain fatty acids and inulin, but not guar gum, prevent diet-induced obesity and insulin resistance through differential mechanisms in mice. Sci Rep 7:6109. https://doi.org/10.1038/s41598-017-06447-x
Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F (2016) From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 165:1332–1345. https://doi.org/10.1016/j.cell.2016.05.041
Neyrinck AM, Possemiers S, Druart C, Van de Wiele T, De Backer F, Cani PD, Larondelle Y, Delzenne NM (2011) Prebiotic effects of wheat arabinoxylan related to the increase in bifidobacteria, roseburia and bacteroides/prevotella in diet-induced obese mice. PLoS ONE 6:e20944. https://doi.org/10.1371/journal.pone.0020944
Neyrinck AM, Possemiers S, Verstraete W, De Backer F, Cani PD, Delzenne NM (2012) Dietary modulation of clostridial cluster XIVa gut bacteria (Roseburia spp.) by chitin-glucan fiber improves host metabolic alterations induced by high-fat diet in mice. J Nutr Biochem 23:51–59. https://doi.org/10.1016/j.jnutbio.2010.10.008
Islam KB, Fukiya S, Hagio M, Fujii N, Ishizuka S, Ooka T, Ogura Y, Hayashi T, Yokota A (2011) Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology 141:1773–1781. https://doi.org/10.1053/j.gastro.2011.07.046
Kang DJ, Hylemon PB, Gillevet PM, Sartor RB, Betrapally NS, Kakiyama G, Sikaroodi M, Takei H, Nittono H, Zhou H, Pandak WM, Yang J, Jiao C, Li X, Lippman HR, Heuman DM, Bajaj JS (2017) Gut microbial composition can differentially regulate bile acid synthesis in humanized mice. Hepatol Commun 1(1):61–70. https://doi.org/10.1002/hep4.1020
Sayin SI, Wahlström A, Felin J, Jäntti S, Marschall HU, Bamberg K, Angelin B, Hyötyläinen T, Orešič M, Bäckhed F (2013) Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab 17(2):225–235. https://doi.org/10.1016/j.cmet.2013.01.003
Degirolamo C, Rainaldi S, Bovenga F, Murzilli S, Moschetta A (2014) Microbiota modification with probiotics induces hepatic bile acid synthesis via downregulation of the Fxr-Fgf15 axis in mice. Cell Rep 7(1):12–18. https://doi.org/10.1016/j.celrep.2014.02.032
Acknowledgements
This research funding was supported by the Hokuto Corporation. We thank Louise Adam, ELS(D), from Edanz Group for editing a draft of this manuscript. We appreciate Takamitsu Shimizu from Hokuto Corporation for providing valuable advice on microbiota analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Two of the authors (KM and MH) are salaried employees of Hokuto Corporation. The remaining authors have no conflict of interest to disclose.
Rights and permissions
About this article
Cite this article
Nakahara, D., Nan, C., Mori, K. et al. Effect of mushroom polysaccharides from Pleurotus eryngii on obesity and gut microbiota in mice fed a high-fat diet. Eur J Nutr 59, 3231–3244 (2020). https://doi.org/10.1007/s00394-019-02162-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-019-02162-7