Abstract
Background
This study assesses survival improvement across a decade (2004–2013) among patients with metastatic gastrointestinal cancers in a real-world setting.
Methods
Surveillance, Epidemiology and End Results (SEER) database was accessed and patients with metastatic gastrointestinal carcinomas who have received any form of systemic therapy were included. Patients were grouped into three cohorts based on the year of diagnosis (cohort-1: 2004–2006; cohort-2: 2008–2010; cohort-3: 2012–2013). Overall survival was compared among the three cohorts for each disease site using Kaplan-Meier survival estimates.
Results
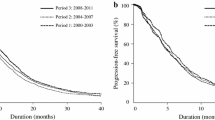
A total of 54,992 patients with metastatic gastrointestinal cancers were included in the current analysis. Using Kaplan-Meier survival comparison for the three temporal cohorts, the following survival observations were noted: for patients with metastatic esophageal adenocarcinoma: median survival for cohort-1: 8 months, cohort-2: 9 months, cohort-3: 9 months; P < 0.001; for patients with metastatic esophageal squamous cell carcinoma: median survival cohort-1: 8 months, cohort-2: 8 months, cohort-3: 8 months; P = 0.689; for patients with metastatic gastric adenocarcinoma: median survival for cohort-1: 8 months, for cohort-2: 9 months, for cohort-3: 9 months; P < 0.001; for patients with metastatic colorectal carcinoma: median overall survival for each of the three cohorts: 21 months; P = 0.131; for patients with metastatic pancreatic carcinoma: median survival for cohort-1: 5 months, cohort-2: 5 months, cohort-3: 6 months; P < 0.001; for patients with metastatic hepatocellular carcinoma: median survival of 5 months for each of the three cohorts; P = 0.534); and for patients with metastatic biliary carcinomas: median survival for cohort-1: 7 months, cohort-2: 7 months, cohort-3: 8 months; P = 0.031).
Conclusion
Limited (if any) survival improvement has been observed among patients with metastatic gastrointestinal carcinomas treated with systemic therapy in the decade from 2004 to 2013.




Similar content being viewed by others
References
Fitzmaurice C, Abate D, Abbasi N, Abbastabar H, Abd-Allah F, Abdel-Rahman O et al Global, Regional, and National Cancer Incidence, Mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: A systematic analysis for the global burden of disease study. JAMA Oncol 2019
Hochster HS, O'Reilly EM, Ajani JA, Venook AP (2007) Section III: Treatment of advanced gastrointestinal cancers. Gastrointest Cancer Res 1(6 Suppl):S8–S12
Kennedy-Martin T, Curtis S, Faries D, Robinson S, Johnston J (2015) A literature review on the representativeness of randomized controlled trial samples and implications for the external validity of trial results. Trials 16:495
He Z, Chen Z, George T, Lipori G, Bian J (2016) Assessing the population representativeness of colorectal cancer treatment clinical trials. 2970–3 p
Ford JG, Howerton MW, Lai GY, Gary TL, Bolen S, Gibbons MC et al (2008) Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer. 112(2):228–242
Torgeson A, Tao R, Garrido-Laguna I, Willen B, Dursteler A, Lloyd S (2018) Large database utilization in health outcomes research in pancreatic cancer: an update. J Gastrointest Oncol 9(6):996–1004
Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 18 Regs Custom Data (with additional treatment fields), Nov 2018 Sub (1975–2016 varying) - linked to county attributes - total U.S., 1969–2017 counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2019, based on the November 2018 submission
Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, Middleton G, Daniel F, Oates J, Norman AR, Upper Gastrointestinal Clinical Studies Group of the National Cancer Research Institute of the United Kingdom (2008) Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 358(1):36–46
Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y et al (2011) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364(19):1817–1825
Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M et al (2013) Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 369(18):1691–1703
Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, Roughton M, Bridgewater J, ABC-02 Trial Investigators (2010) Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 362(14):1273–1281
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J, SHARP Investigators Study Group (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359(4):378–390
Douillard J-Y, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J, Rivera F, Kocákova I, Ruff P, Błasińska-Morawiec M, Šmakal M, Canon JL, Rother M, Oliner KS, Wolf M, Gansert J (2010) Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol 28(31):4697–4705
Donia M, Hansen SW, Svane IM (2019) Real-world evidence to guide healthcare policies in oncology. Oncotarget 10(44)
Canoui-Poitrine F, Lievre A, Dayde F, Lopez-Trabada-Ataz D, Baumgaertner I, Dubreuil O et al (2019) Inclusion of older patients with cancer in clinical trials: the SAGE prospective multicenter cohort survey. Oncologist.
Abdel-Rahman O, Karachiwala H (2019) Impact of age on toxicity and efficacy of 5-FU-based combination chemotherapy among patients with metastatic colorectal cancer; a pooled analysis of five randomized trials. Int J Color Dis 34(10):1741–1747
Abdel-Rahman O (2019) ECOG performance score 0 versus 1: impact on efficacy and safety of first-line 5-FU-based chemotherapy among patients with metastatic colorectal cancer included in five randomized trials. Int J Color Dis 34(12):2143–2150
Unger JM, Hershman DL, Fleury ME, Vaidya R (2019) Association of patient comorbid conditions with cancer clinical trial participation. JAMA Oncol 5(3):326–333
Abdel-Rahman O (2018) Impact of baseline characteristics on outcomes of advanced HCC patients treated with sorafenib: a secondary analysis of a phase III study. J Cancer Res Clin Oncol 144(5):901–908
https://www.fda.gov/regulatory-information/search-fda-guidance-documents/clinical-trial-endpoints-approval-cancer-drugs-and-biologics. Last accessed on 24/11/2019
Kemp R, Prasad V (2017) Surrogate endpoints in oncology: when are they acceptable for regulatory and clinical decisions, and are they currently overused? BMC Med 15(1):134
Hilal T, Sonbol MB, Prasad V (2019) Analysis of control arm quality in randomized clinical trials leading to anticancer drug approval by the US Food and Drug Administration. JAMA Oncol. 5(6):887–892
Abdel-Rahman O (2019) Outcomes of non-metastatic colon cancer patients in relationship to socioeconomic status: an analysis of SEER census tract-level socioeconomic database. Int J Clin Oncol 24(12):1582–1587
Chandrasoma PT (2018) Chapter 12 - esophageal adenocarcinoma. In: Chandrasoma PT (ed) GERD. Academic Press, pp 341–389
Holowatyj AN, Gigic B, Herpel E, Scalbert A, Schneider M, Ulrich CM (2019) Distinct molecular phenotype of sporadic colorectal cancers among young patients based on multi-omics analysis. Gastroenterology
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abdel-Rahman, O. A 10-year review of survival among patients with metastatic gastrointestinal cancers: a population-based study. Int J Colorectal Dis 35, 911–920 (2020). https://doi.org/10.1007/s00384-020-03568-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-020-03568-0