Abstract
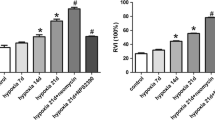
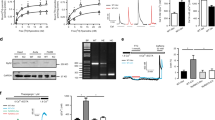
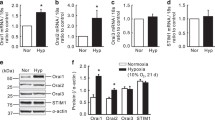
Pulmonary arterial hypertension (PAH) is a progressive disease associated with vasoconstriction and remodeling. Intracellular Ca2+ signaling regulates the contraction of pulmonary arteries and the proliferation of pulmonary arterial smooth muscle cells (PASMCs); however, it is not clear which molecules related to Ca2+ signaling contribute to the progression of PAH. In this study, we found the specific expression of type 2 inositol 1,4,5-trisphosphate receptor (IP3R2), which is an intracellular Ca2+ release channel, on the sarco/endoplasmic reticulum in mouse PASMCs, and demonstrated its inhibitory role in the progression of PAH using a chronic hypoxia-induced PAH mouse model. After chronic hypoxia exposure, IP3R2−/− mice exhibited the significant aggravation of PAH, as determined by echocardiography and right ventricular hypertrophy, with significantly greater medial wall thickness by immunohistochemistry than that of wild-type mice. In IP3R2−/− murine PASMCs with chronic hypoxia, a TUNEL assay revealed the significant suppression of apoptosis, whereas there was no significant change in proliferation. Thapsigargin-induced store-operated Ca2+ entry (SOCE) was significantly enhanced in IP3R2−/− PASMCs in both normoxia and hypoxia based on in vitro fluorescent Ca2+ imaging. Furthermore, the enhancement of SOCE in IP3R2−/− PASMCs was remarkably suppressed by the addition of DPB162-AE, an inhibitor of the stromal-interacting molecule (STIM)–Orai complex which is about 100 times more potent than 2-APB. Our results indicate that IP3R2 may inhibit the progression of PAH by promoting apoptosis and inhibiting SOCE via the STIM–Orai pathway in PASMCs. These findings suggest a previously undetermined role of IP3R in the development of PAH and may contribute to the development of targeted therapies.







Similar content being viewed by others
References
Thenappan T, Ormiston ML, Ryan JJ, Archer SL (2018) Pulmonary arterial hypertension: pathogenesis and clinical management. BMJ 360:j5492
Kuhr FK, Smith KA, Song MY, Levitan I, Yuan JX (2012) New mechanisms of pulmonary arterial hypertension: role of Ca(2)(+) signaling. Am J Physiol Heart Circ Physiol 302:H1546–H1562
Archer S, Rich S (2000) Primary pulmonary hypertension: a vascular biology and translational research "Work in progress". Circulation 102:2781–2791
Lin MJ, Leung GP, Zhang WM, Yang XR, Yip KP, Tse CM, Sham JS (2004) Chronic hypoxia-induced upregulation of store-operated and receptor-operated Ca2+ channels in pulmonary arterial smooth muscle cells: a novel mechanism of hypoxic pulmonary hypertension. Circ Res 95:496–505
Reyes RV, Castillo-Galan S, Hernandez I, Herrera EA, Ebensperger G, Llanos AJ (2018) Revisiting the role of TRP, Orai, and ASIC channels in the pulmonary arterial response to hypoxia. Front Physiol 9:486
Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G (1999) Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature 397:259–263
Avila-Medina J, Mayoral-Gonzalez I, Dominguez-Rodriguez A, Gallardo-Castillo I, Ribas J, Ordonez A, Rosado JA, Smani T (2018) The Complex role of store operated calcium entry pathways and related proteins in the function of cardiac, skeletal and vascular smooth muscle cells. Front Physiol 9:257
Putney JW (2018) Forms and functions of store-operated calcium entry mediators, STIM and Orai. Adv Biol Regul 68:88–96
Mikoshiba K (2015) Role of IP3 receptor signaling in cell functions and diseases. Adv Biol Regul 57:217–227
Berridge MJ (2016) The inositol trisphosphate/calcium signaling pathway in health and disease. Physiol Rev 96:1261–1296
Wojcikiewicz RJ (1995) Type I, II, and III inositol 1,4,5-trisphosphate receptors are unequally susceptible to down-regulation and are expressed in markedly different proportions in different cell types. J Biol Chem 270:11678–11683
Klar J, Hisatsune C, Baig SM, Tariq M, Johansson AC, Rasool M, Malik NA, Ameur A, Sugiura K, Feuk L, Mikoshiba K, Dahl N (2014) Abolished InsP3R2 function inhibits sweat secretion in both humans and mice. J Clin Invest 124:4773–4780
Inaba T, Hisatsune C, Sasaki Y, Ogawa Y, Ebisui E, Ogawa N, Matsui M, Takeuchi T, Mikoshiba K, Tsubota K (2014) Mice lacking inositol 1,4,5-trisphosphate receptors exhibit dry eye. PLoS One 9:e99205
Futatsugi A, Nakamura T, Yamada MK, Ebisui E, Nakamura K, Uchida K, Kitaguchi T, Takahashi-Iwanaga H, Noda T, Aruga J, Mikoshiba K (2005) IP3 receptor types 2 and 3 mediate exocrine secretion underlying energy metabolism. Science 309:2232–2234
Nakayama H, Bodi I, Maillet M, DeSantiago J, Domeier TL, Mikoshiba K, Lorenz JN, Blatter LA, Bers DM, Molkentin JD (2010) The IP3 receptor regulates cardiac hypertrophy in response to select stimuli. Circ Res 107:659–666
Uchida K, Aramaki M, Nakazawa M, Yamagishi C, Makino S, Fukuda K, Nakamura T, Takahashi T, Mikoshiba K, Yamagishi H (2010) Gene knock-outs of inositol 1,4,5-trisphosphate receptors types 1 and 2 result in perturbation of cardiogenesis. PLoS One 5:e12500
Nakazawa M, Uchida K, Aramaki M, Kodo K, Yamagishi C, Takahashi T, Mikoshiba K, Yamagishi H (2011) Inositol 1,4,5-trisphosphate receptors are essential for the development of the second heart field. J Mol Cell Cardiol 51:58–66
Uchida K, Nakazawa M, Yamagishi C, Mikoshiba K, Yamagishi H (2016) Type 1 and 3 inositol trisphosphate receptors are required for extra-embryonic vascular development. Dev Biol 418:89–97
Narayanan D, Adebiyi A, Jaggar JH (2012) Inositol trisphosphate receptors in smooth muscle cells. Am J Physiol Heart Circ Physiol 302:H2190–H2210
Kawakami T, Kanazawa H, Satoh T, Ieda M, Ieda Y, Kimura K, Mochizuki H, Shimada T, Yokoyama C, Ogawa S, Tanabe T, Fukuda K (2007) AAV-PGIS gene transfer improves hypoxia-induced pulmonary hypertension in mice. Biochem Biophys Res Commun 363:656–661
Levy PT, Patel MD, Groh G, Choudhry S, Murphy J, Holland MR, Hamvas A, Grady MR, Singh GK (2016) Pulmonary artery acceleration time provides a reliable estimate of invasive pulmonary hemodynamics in children. J Am Soc Echocardiogr 29:1056–1065
Gomez O, Okumura K, Honjo O, Sun M, Ishii R, Bijnens B, Friedberg MK (2018) Heart rate reduction improves biventricular function and interactions in experimental pulmonary hypertension. Am J Physiol Heart Circ Physiol 314:H542–H551
Yamagishi H, Olson EN, Srivastava D (2000) The basic helix-loop-helix transcription factor, dHAND, is required for vascular development. J Clin Investig 105:261–270
Yokoyama U, Minamisawa S, Adachi-Akahane S, Akaike T, Naguro I, Funakoshi K, Iwamoto M, Nakagome M, Uemura N, Hori H, Yokota S, Ishikawa Y (2006) Multiple transcripts of Ca2+ channel alpha1-subunits and a novel spliced variant of the alpha1C-subunit in rat ductus arteriosus. Am J Physiol Heart Circ Physiol 290:H1660–H1670
Nakamura K, Hamada K, Terauchi A, Matsui M, Nakamura T, Okada T, Mikoshiba K (2013) Distinct roles of M1 and M3 muscarinic acetylcholine receptors controlling oscillatory and non-oscillatory [Ca2+] i increase. Cell Calcium 54:111–119
Miyauchi T, Yamamoto H, Abe Y, Yoshida GJ, Rojek A, Sohara E, Uchida S, Nielsen S, Yasui M (2015) Dynamic subcellular localization of aquaporin-7 in white adipocytes. FEBS Lett 589:608–614
Jozsef L, Tashiro K, Kuo A, Park EJ, Skoura A, Albinsson S, Rivera-Molina F, Harrison KD, Iwakiri Y, Toomre D, Sessa WC (2014) Reticulon 4 is necessary for endoplasmic reticulum tubulation, STIM1-Orai1 coupling, and store-operated calcium entry. J Biol Chem 289:9380–9395
Goto J, Suzuki AZ, Ozaki S, Matsumoto N, Nakamura T, Ebisui E, Fleig A, Penner R, Mikoshiba K (2010) Two novel 2-aminoethyl diphenylborinate (2-APB) analogues differentially activate and inhibit store-operated Ca(2+) entry via STIM proteins. Cell Calcium 47:1–10
Hendron E, Wang X, Zhou Y, Cai X, Goto J, Mikoshiba K, Baba Y, Kurosaki T, Wang Y, Gill DL (2014) Potent functional uncoupling between STIM1 and Orai1 by dimeric 2-aminodiphenyl borinate analogs. Cell Calcium 56:482–492
Klinger JR, Warburton RR, Pietras LA, Smithies O, Swift R, Hill NS (1999) Genetic disruption of atrial natriuretic peptide causes pulmonary hypertension in normoxic and hypoxic mice. Am J Physiol 276:L868–L874
Ogawa A, Firth AL, Smith KA, Maliakal MV, Yuan JX (2012) PDGF enhances store-operated Ca2+ entry by upregulating STIM1/Orai1 via activation of Akt/mTOR in human pulmonary arterial smooth muscle cells. Am J Physiol Cell Physiol 302:C405–C411
Yadav VR, Song T, Mei L, Joseph L, Zheng YM, Wang YX (2018) PLCgamma1-PKCepsilon-IP3R1 signaling plays an important role in hypoxia-induced calcium response in pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 314:L724–L735
Joseph SK, Hajnoczky G (2007) IP3 receptors in cell survival and apoptosis: Ca2+ release and beyond. Apoptosis 12:951–968
Lencesova L, Krizanova O (2012) IP(3) receptors, stress and apoptosis. Gen Physiol Biophys 31:119–130
Akl H, Monaco G, La Rovere R, Welkenhuyzen K, Kiviluoto S, Vervliet T, Molgo J, Distelhorst CW, Missiaen L, Mikoshiba K, Parys JB, De Smedt H, Bultynck G (2013) IP3R2 levels dictate the apoptotic sensitivity of diffuse large B-cell lymphoma cells to an IP3R-derived peptide targeting the BH4 domain of Bcl-2. Cell Death Dis 4:e632
Zhong F, Harr MW, Bultynck G, Monaco G, Parys JB, De Smedt H, Rong YP, Molitoris JK, Lam M, Ryder C, Matsuyama S, Distelhorst CW (2011) Induction of Ca(2)+-driven apoptosis in chronic lymphocytic leukemia cells by peptide-mediated disruption of Bcl-2-IP3 receptor interaction. Blood 117:2924–2934
Yu Y, Fantozzi I, Remillard CV, Landsberg JW, Kunichika N, Platoshyn O, Tigno DD, Thistlethwaite PA, Rubin LJ, Yuan JX (2004) Enhanced expression of transient receptor potential channels in idiopathic pulmonary arterial hypertension. Proc Natl Acad Sci USA 101:13861–13866
Song MY, Makino A, Yuan JX (2011) STIM2 contributes to enhanced store-operated Ca entry in pulmonary artery smooth muscle cells from patients with idiopathic pulmonary arterial hypertension. Pulm Circ 1:84–94
Pan Z, Brotto M, Ma J (2014) Store-operated Ca2+ entry in muscle physiology and diseases. BMB Rep 47:69–79
Venkatachalam K, van Rossum DB, Patterson RL, Ma HT, Gill DL (2002) The cellular and molecular basis of store-operated calcium entry. Nat Cell Biol 4:E263–E272
Ng LC, O'Neill KG, French D, Airey JA, Singer CA, Tian H, Shen XM, Hume JR (2012) TRPC1 and Orai1 interact with STIM1 and mediate capacitative Ca(2+) entry caused by acute hypoxia in mouse pulmonary arterial smooth muscle cells. Am J Physiol Cell Physiol 303:C1156–C1172
Lur G, Sherwood MW, Ebisui E, Haynes L, Feske S, Sutton R, Burgoyne RD, Mikoshiba K, Petersen OH, Tepikin AV (2011) InsP(3)receptors and Orai channels in pancreatic acinar cells: co-localization and its consequences. Biochem J 436:231–239
Acknowledgements
The authors thank K. Fukuda who helped to develop the mouse model system of pulmonary hypertension induced by hypoxia; U. Yokoyama for the preparation of primary culture of pulmonary artery smooth muscle cells; M. Yasui for Ca2+ imaging with confocal microscopy. We thank Olympus Corp. for the support in Ca2+ imaging. The authors also thank A. Kuramochi and M. Suzuki for technical support, and members of the Division of Pediatric Cardiology in Keio University School of Medicine for helpful discussion. This work was supported by the Acterion Academia Prize 2015 to A.S. And it was also supported by Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan to K.U. and H.Y. (Grant numbers JP19390288, JP19790740, JP21791004, JP23591583, JP26461619, JP17K10153, and JP16H05359).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Shibata, A., Uchida, K., Kodo, K. et al. Type 2 inositol 1,4,5-trisphosphate receptor inhibits the progression of pulmonary arterial hypertension via calcium signaling and apoptosis. Heart Vessels 34, 724–734 (2019). https://doi.org/10.1007/s00380-018-1304-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-018-1304-4