Abstract
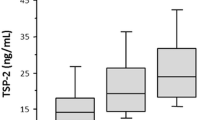
Serum levels of the soluble urokinase-type plasminogen activator receptor (suPAR) reflect immune and inflammatory activation, and are shown to be associated with cardiovascular outcomes. We herein investigated the potential association between suPAR and left ventricular diastolic dysfunction among patients with preserved left ventricular ejection fraction (LVEF) and sinus rhythm. Among 291 patients who had sinus rhythm and an LVEF of ≥50% enrolled in the study, 26 (8.9%) were considered to have diastolic dysfunction. Patients with diastolic dysfunction had lower estimated glomerular filtration rate (eGFR), and higher systolic blood pressure (BPs), BNP, C-reactive protein, and suPAR than those without diastolic dysfunction. As compared with the first suPAR quartile, the fourth suPAR quartile was significantly associated with both diastolic dysfunction with an odds ratio of 8.95 [95% confidence interval (CI), 1.04–77.0, P < 0.05] after adjusting for sex, age, BPs log(eGFR), CRP, and diuretic use. On the other hand, receiver-operating characteristic curve (ROC) analysis showed that addition of log(suPAR) to the combination of age, sex, and log(eGFR), CRP, and diuretic use did not significantly improve the prediction of diastolic dysfunction. Among cardiac patients with preserved LVEF, serum suPAR was associated with diastolic dysfunction independent of confounding factors by logistic regression analysis. However, according to the ROC analysis, the utility of suPAR as a biomarker for diastolic dysfunction may be limited from a clinical point of view.



Similar content being viewed by others
References
Del Rosso M, Margheri F, Serrati S, Chilla A, Laurenzana A, Fibbi G (2011) The urokinase receptor system, a key regulator at the intersection between inflammation, immunity, and coagulation. Curr Pharm Des 17:1924–1943
Su SC, Lin CW, Yang WE, Fan WL, Yang SF (2016) The urokinase-type plasminogen activator (uPA) system as a biomarker and therapeutic target in human malignancies. Expert Opin Ther Targets 20:551–566
Sandquist M, Wong HR (2014) Biomarkers of sepsis and their potential value in diagnosis, prognosis and treatment. Expert Rev Clin Immunol 10:1349–1356
Ni W, Han Y, Zhao J, Cui J, Wang K, Wang R, Liu Y (2016) Serum soluble urokinase-type plasminogen activator receptor as a biological marker of bacterial infection in adults: a systematic review and meta-analysis. Sci Rep 6:39481
Zeng M, Chang M, Zheng H, Li B, Chen Y, He W, Huang C (2016) Clinical value of soluble urokinase-type plasminogen activator receptor in the diagnosis, prognosis, and therapeutic guidance of sepsis. Am J Emerg Med 34:375–380
Lomholt AF, Christensen IJ, Hoyer-Hansen G, Nielsen HJ (2010) Prognostic value of intact and cleaved forms of the urokinase plasminogen activator receptor in a retrospective study of 518 colorectal cancer patients. Acta Oncol 49:805–811
Zimmermann HW, Koch A, Seidler S, Trautwein C, Tacke F (2012) Circulating soluble urokinase plasminogen activator is elevated in patients with chronic liver disease, discriminates stage and aetiology of cirrhosis and predicts prognosis. Liver Int 32:500–509
Persson M, Engstrom G, Bjorkbacka H, Hedblad B (2012) Soluble urokinase plasminogen activator receptor in plasma is associated with incidence of CVD. Results from the malmo diet and cancer study. Atherosclerosis 220:502–505
Eapen DJ, Manocha P, Ghasemzadeh N, Patel RS, Al Kassem H, Hammadah M, Veledar E, Le NA, Pielak T, Thorball CW, Velegraki A, Kremastinos DT, Lerakis S, Sperling L, Quyyumi AA (2014) Soluble urokinase plasminogen activator receptor level is an independent predictor of the presence and severity of coronary artery disease and of future adverse events. J Am Heart Assoc 3:e001118
Sorensen MH, Gerke O, Eugen-Olsen J, Munkholm H, Lambrechtsen J, Sand NP, Mickley H, Rasmussen LM, Olsen MH, Diederichsen A (2014) Soluble urokinase plasminogen activator receptor is in contrast to high-sensitive C-reactive-protein associated with coronary artery calcifications in healthy middle-aged subjects. Atherosclerosis 237:60–66
Lyngbaek S, Marott JL, Sehestedt T, Hansen TW, Olsen MH, Andersen O, Linneberg A, Haugaard SB, Eugen-Olsen J, Hansen PR, Jeppesen J (2013) Cardiovascular risk prediction in the general population with use of suPAR, CRP, and Framingham risk score. Int J Cardiol 167:2904–2911
Borne Y, Persson M, Melander O, Smith JG, Engstrom G (2014) Increased plasma level of soluble urokinase plasminogen activator receptor is associated with incidence of heart failure but not atrial fibrillation. Eur J Heart Fail 16:377–383
Fujita SI, Tanaka S, Maeda D, Morita H, Fujisaka T, Takeda Y, Ito T, Ishizaka N (2017) Serum soluble urokinase-type plasminogen activator receptor is associated with low left ventricular ejection fraction and elevated plasma brain-type natriuretic peptide level. PLoS ONE 12:e0170546
Matsubara J, Sugiyama S, Nozaki T, Sugamura K, Konishi M, Ohba K, Matsuzawa Y, Akiyama E, Yamamoto E, Sakamoto K, Nagayoshi Y, Kaikita K, Sumida H, Kim-Mitsuyama S, Ogawa H (2011) Pentraxin 3 is a new inflammatory marker correlated with left ventricular diastolic dysfunction and heart failure with normal ejection fraction. J Am Coll Cardiol 57:861–869
Yamamoto E, Hirata Y, Tokitsu T, Kusaka H, Tabata N, Tsujita K, Yamamuro M, Kaikita K, Watanabe H, Hokimoto S, Maruyama T, Ogawa H (2016) The clinical significance of plasma neopterin in heart failure with preserved left ventricular ejection fraction. ESC Heart Fail 3:53–59
Theilade S, Rossing P, Eugen-Olsen J, Jensen JS, Jensen MT (2016) suPAR level is associated with myocardial impairment assessed with advanced echocardiography in patients with type 1 diabetes with normal ejection fraction and without known heart disease or end-stage renal disease. Eur J Endocrinol 174:745–753
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A (2009) Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53:982–992
Devereux RB, Reichek N (1977) Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation 55:613–618
Wachtell K, Bella JN, Liebson PR, Gerdts E, Dahlof B, Aalto T, Roman MJ, Papademetriou V, Ibsen H, Rokkedal J, Devereux RB (2000) Impact of different partition values on prevalences of left ventricular hypertrophy and concentric geometry in a large hypertensive population: the LIFE study. Hypertension 35:6–12
Tanaka S, Fujita S, Kizawa S, Morita H, Ishizaka N (2016) Association between FGF23, alpha-Klotho, and cardiac abnormalities among patients with various chronic kidney disease stages. PLoS ONE 11:e0156860
Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite-Moreira AF, Borbely A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL (2007) How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J 28:2539–2550
Devereux RB, James GD, Pickering TG (1993) What is normal blood pressure? Comparison of ambulatory pressure level and variability in patients with normal or abnormal left ventricular geometry. Am J Hypertens 6:211S–215S
Mitter SS, Shah SJ, Thomas JD (2017) A test in context: E/A and E/e′ to assess diastolic dysfunction and LV filling pressure. J Am Coll Cardiol 69:1451–1464
Sato W, Kosaka T, Koyama T, Ishida M, Iino K, Watanabe H, Ito H (2013) Impaired renal function is a major determinant of left ventricular diastolic dysfunction: assessment by stress myocardial perfusion imaging. Ann Nucl Med 27:729–736
Unger ED, Dubin RF, Deo R, Daruwalla V, Friedman JL, Medina C, Beussink L, Freed BH, Shah SJ (2016) Association of chronic kidney disease with abnormal cardiac mechanics and adverse outcomes in patients with heart failure and preserved ejection fraction. Eur J Heart Fail 18:103–112
Ter Maaten JM, Damman K, Verhaar MC, Paulus WJ, Duncker DJ, Cheng C, van Heerebeek L, Hillege HL, Lam CS, Navis G, Voors AA (2016) Connecting heart failure with preserved ejection fraction and renal dysfunction: the role of endothelial dysfunction and inflammation. Eur J Heart Fail 18:588–598
Gromadzinski L, Januszko-Giergielewicz B, Pruszczyk P (2014) Hypocalcemia is related to left ventricular diastolic dysfunction in patients with chronic kidney disease. J Cardiol 63:198–204
Nogi S, Fujita S, Okamoto Y, Kizawa S, Morita H, Ito T, Sakane K, Sohmiya K, Hoshiga M, Ishizaka N (2015) Serum uric acid is associated with cardiac diastolic dysfunction among women with preserved ejection fraction. Am J Physiol Heart Circ Physiol 309:H986–H994
Okamoto Y, Fujita S, Morita H, Kizawa S, Ito T, Sakane K, Sohmiya K, Hoshiga M, Ishizaka N (2016) Association between circulating FGF23, alpha-Klotho, and left ventricular diastolic dysfunction among patients with preserved ejection fraction. Heart Vessels 31:66–73
Hayek SS, Sever S, Ko YA, Trachtman H, Awad M, Wadhwani S, Altintas MM, Wei C, Hotton AL, French AL, Sperling LS, Lerakis S, Quyyumi AA, Reiser J (2015) Soluble urokinase receptor and chronic kidney disease. N Engl J Med 373:1916–1925
Suleiman M, Khatib R, Agmon Y, Mahamid R, Boulos M, Kapeliovich M, Levy Y, Beyar R, Markiewicz W, Hammerman H, Aronson D (2006) Early inflammation and risk of long-term development of heart failure and mortality in survivors of acute myocardial infarction predictive role of C-reactive protein. J Am Coll Cardiol 47:962–968
Dick SA, Epelman S (2016) Chronic heart failure and inflammation: what do we really know? Circ Res 119:159–176
Van Tassell BW, Arena R, Biondi-Zoccai G, McNair Canada J, Oddi C, Abouzaki NA, Jahangiri A, Falcao RA, Kontos MC, Shah KB, Voelkel NF, Dinarello CA, Abbate A (2014) Effects of interleukin-1 blockade with anakinra on aerobic exercise capacity in patients with heart failure and preserved ejection fraction (from the D-HART pilot study). Am J Cardiol 113:321–327
Glezeva N, Voon V, Watson C, Horgan S, McDonald K, Ledwidge M, Baugh J (2015) Exaggerated inflammation and monocytosis associate with diastolic dysfunction in heart failure with preserved ejection fraction: evidence of M2 macrophage activation in disease pathogenesis. J Card Fail 21:167–177
Tang L, Han X (2013) The urokinase plasminogen activator system in breast cancer invasion and metastasis. Biomed Pharmacother 67:179–182
Backes Y, van der Sluijs KF, Mackie DP, Tacke F, Koch A, Tenhunen JJ, Schultz MJ (2012) Usefulness of suPAR as a biological marker in patients with systemic inflammation or infection: a systematic review. Intensive Care Med 38:1418–1428
Hodges GW, Bang CN, Wachtell K, Eugen-Olsen J, Jeppesen JL (2015) suPAR: a new biomarker for cardiovascular disease? Can J Cardiol 31:1293–1302
Mekonnen G, Corban MT, Hung OY, Eshtehardi P, Eapen DJ, Al-Kassem H, Rasoul-Arzrumly E, Gogas BD, McDaniel MC, Pielak T, Thorball CW, Sperling L, Quyyumi AA, Samady H (2015) Plasma soluble urokinase-type plasminogen activator receptor level is independently associated with coronary microvascular function in patients with non-obstructive coronary artery disease. Atherosclerosis 239:55–60
Lyngbaek S, Sehestedt T, Marott JL, Hansen TW, Olsen MH, Andersen O, Linneberg A, Madsbad S, Haugaard SB, Eugen-Olsen J, Jeppesen J (2013) CRP and suPAR are differently related to anthropometry and subclinical organ damage. Int J Cardiol 167:781–785
Paulus WJ, Tschope C (2013) A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 62:263–271
Namba T, Masaki N, Matsuo Y, Sato A, Kimura T, Horii S, Yasuda R, Yada H, Kawamura A, Takase B, Adachi T (2016) Arterial stiffness is significantly associated with left ventricular diastolic dysfunction in patients with cardiovascular disease. Int Heart J 57:729–735
Franssen C, Chen S, Unger A, Korkmaz HI, De Keulenaer GW, Tschope C, Leite-Moreira AF, Musters R, Niessen HW, Linke WA, Paulus WJ, Hamdani N (2016) Myocardial microvascular inflammatory endothelial activation in heart failure with preserved ejection fraction. JACC Heart Fail 4:312–324
du Plooy CS, Kruger R, Huisman HW, Rasmussen LM, Eugen-Olsen J, Schutte AE (2015) Extracellular matrix biomarker, fibulin-1 and its association with soluble uPAR in a bi-ethnic South African population: the SAfrEIC study. Heart Lung Circ 24:298–305
Osawa K, Miyoshi T, Oe H, Sato S, Nakamura K, Kohno K, Morita H, Kanazawa S, Ito H (2016) Association between coronary artery calcification and left ventricular diastolic dysfunction in elderly people. Heart Vessels 31:499–507
Suzuki S, Sagara K, Otsuka T, Matsuno S, Funada R, Uejima T, Oikawa Y, Koike A, Nagashima K, Kirigaya H, Yajima J, Sawada H, Aizawa T, Yamashita T (2012) Gender-specific relationship between serum uric acid level and atrial fibrillation prevalence. Circ J 76:607–611
Ndrepepa G, Cassese S, Braun S, Fusaro M, King L, Tada T, Schomig A, Kastrati A, Schmidt R (2013) A gender-specific analysis of association between hyperuricaemia and cardiovascular events in patients with coronary artery disease. Nutr Metab Cardiovasc Dis 23:1195–1201
Onoue Y, Izumiya Y, Hanatani S, Kimura Y, Araki S, Sakamoto K, Yamamoto E, Tsujita K, Tanaka T, Yamamuro M, Kojima S, Kaikita K, Hokimoto S, Ogawa H (2016) Fragmented QRS complex is a diagnostic tool in patients with left ventricular diastolic dysfunction. Heart Vessels 31:563–567
Acknowledgements
This work was supported in part by the Grants-in-Aid for Scientific Research from the Ministry of Education, Science, and Culture of Japan (No. 15K09106). We are highly appreciative of Chieko Ohta, Yumiko Ohgami, and Megumi Hashimoto for their excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Fujisaka, T., Fujita, Si., Maeda, D. et al. Association between suPAR and cardiac diastolic dysfunction among patients with preserved ejection fraction. Heart Vessels 32, 1327–1336 (2017). https://doi.org/10.1007/s00380-017-1002-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-017-1002-7