Abstract
Objectives
To propose a machine learning–based methodology for the creation of radiation dose maps and the prediction of patient-specific organ/tissue doses associated with head CT examinations.
Methods
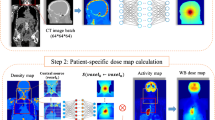
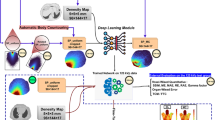
CT data were collected retrospectively for 343 patients who underwent standard head CT examinations. Patient-specific Monte Carlo (MC) simulations were performed to determine the radiation dose distribution to patients’ organs/tissues. The collected CT images and the MC–produced dose maps were processed and used for the training of the deep neural network (DNN) model. For the training and validation processes, data from 231 and 112 head CT examinations, respectively, were used. Furthermore, a software tool was developed to produce dose maps from head CT images using the trained DNN model and to automatically calculate the dose to the brain and cranial bones.
Results
The mean (range) percentage differences between the doses predicted from the DNN model and those provided by MC simulations for the brain, eye lenses, and cranial bones were 4.5% (0–17.7%), 5.7% (0.2–19.0%), and 5.2% (0.1–18.9%), respectively. The graphical user interface of the software offers a user-friendly way for radiation dose/risk assessment. The implementation of the DNN allowed for a 97% reduction in the computational time needed for the dose estimations.
Conclusions
A novel methodology that allows users to develop a DNN model for patient-specific CT dose prediction was developed and implemented. The approach demonstrated herein allows accurate and fast radiation dose estimation for the brain, eye lenses, and cranial bones of patients who undergo head CT examinations and can be used in everyday clinical practice.
Key Points
• The methodology presented herein allows fast and accurate radiation dose estimation for the brain, eye lenses, and cranial bones of patients who undergo head CT examinations and can be implemented in everyday clinical practice.
• The scripts developed in the current study will allow users to train models for the acquisition protocols of their CT scanners, generate dose maps, estimate the doses to the brain and cranial bones, and estimate the lifetime attributable risk of radiation-induced brain cancer.




Similar content being viewed by others
Abbreviations
- CT:
-
Computed tomography
- CTDI:
-
CT dose index
- DNN:
-
Deep neural network
- GUI:
-
Graphical user interface
- HU:
-
Hounsfield unit
- ICRP:
-
International Commission on Radiological Protection
- LAR:
-
Lifetime attributable risk
- MC:
-
Monte Carlo
- ROI:
-
Region of interest
References
Gaudreau K, Thome C, Weaver B, Boreham DR (2020) Cataract formation and low-dose radiation exposure from head computed tomography (CT) scans in Ontario, Canada, 1994-2015. Radiat Res 193:322–330
Brinjikji W, Kallmes DF, Cloft HJ (2015) Rising utilization of CT in adult fall patients. AJR Am J Roentgenol 204:558–562
Kim HG, Lee HJ, Lee SK, Kim HJ, Kim MJ (2017) Head CT: image quality improvement with ASIR-V using a reduced radiation dose protocol for children. Eur Radiol 27:3609–3617
Investigative Report ICES (2007) Enhancing the effectiveness of health care for Ontarians through research Diagnostic Services in Ontario: Descriptive Analysis and Jurisdictional Review. ICES, Toronto, ON
Götz TI, Schmidkonz C, Chen S, Al-Baddai S, Kuwert T, Lang EW (2020) A deep learning approach to radiation dose estimation. Phys Med Biol 65:035007
Lee MS, Hwang D, Kim JH, Lee JS (2019) Deep-dose: a voxel dose estimation method using deep convolutional neural network for personalized internal dosimetry. Sci Rep 9(1):10308
Maier J, Eulig E, Dorn S, Sawall S, Kachelrieß M (2018) Real-time patient-specific CT dose estimation using a deep convolutional neural network. IEEE Nuclear Science Symposium and Medical Imaging Conference Proceedings (NSS/MIC), pp 1–3
Deak P, van Straten M, Shrimpton PC, Zankl M, Kalender WA (2008) Validation of a Monte Carlo tool for patient-specific dose simulations in multi-slice computed tomography. Eur Radiol 18:759–772
Myronakis M, Perisinakis K, Tzedakis A, Gourtsoyianni S, Damilakis J (2009) Evaluation of a patient-specific Monte Carlo software for CT dosimetry. Radiat Prot Dosimetry 133:248–255
Damilakis J, Perisinakis K, Tzedakis A, Papadakis AE, Karantanas A (2010) Radiation dose to the conceptus from multidetector CT during early gestation: a method that allows for variations in maternal body size and conceptus position. Radiology 257:483–489
Lloyd SP (1982) Least squares quantization in PCM. IEEE Trans Inf Theory 28:129–137
Erisoglu M, Calis N, Sakallioglu S (2011) A new algorithm for initial cluster centers in k-means algorithm. Pattern Recognit Lett 32:1701–1705
Pedregosa F, Varoquaux G, Gramfort A et al (2011) Scikit-learn: machine learning in Python. J Mach Learn Res 12:2825–2830
Harris CR, Millman KJ, van der Walt SJ et al (2020) Array programming with NumPy. Nature 585:357–362
Kingma DP, Ba J (2014) Adam: a method for stochastic optimization. Available at: http://arxiv.org/abs/1412.6980.
Srivastava N, Hinton G, Krizhevsky A, Sutskever I, Salakhutdinov R (2014) Dropout: a simple way to prevent neural networks from overfitting. J Mach Learn Res 15:1929–1958
Li L, Jamieson K, DeSalvo G, Rostamizadeh A, Talwalkar A (2018) Hyperband: a novel bandit-based approach to hyperparameter optimization. J Mach Learn Res 18:1–52
Agarap AF (2018) Deep learning using rectified linear units (relu). ArXiv Preprint ArXiv:1803.08375.
National Research Council (2006) Health risks from exposure to low levels of ionizing radiation: BEIR VII Phase 2. The National Academies Press, Washington, DC
Van Rossum G (2020) The Python library reference, release 3.8.2. Python Software Foundation 2020
Schindelin J, Arganda-Carreras I, Frise E et al (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682
Damilakis J (2021) CT dosimetry: what has been achieved and what remains to be done. Invest Radiol 56:62–68
ICRP (2007) The 2007 Recommendations of the International Commission on Radiological Protection. CRP publication 103. Ann ICRP 37:9–34
Harrison JD, Balonov M, Bochud F et al (2021) ICRP Publication 147: use of dose quantities in radiological protection. Ann ICRP 50:9–82
Ria F, Bergantin A, Vai A et al (2017) Awareness of medical radiation exposure among patients: a patient survey as a first step for effective communication of ionizing radiation risks. Phys Med 43:57–62
Sin HK, Wong CS, Huang B, Yiu KL, Wong WL, Chu YCT (2013) Assessing local patients’ knowledge and awareness of radiation dose and risks associated with medical imaging: a questionnaire study. J Med Imaging Radiat Oncol 57:38–44
Schuster AL, Forman HP, Strassle PD, Meyer LT, Connelly SV, Lee CI (2018) Awareness of radiation risks from CT scans among patients and providers and obstacles for informed decision-making. Emerg Radiol 25:41–49
Lee CI, Haims AH, Monico EP, Brink JA, Forman HP (2004) Diagnostic CT scans: assessment of patient, physician, and radiologist awareness of radiation dose and possible risks. Radiology 231:393–398
Peng Z, Fang X, Yan P et al (2020) A method of rapid quantification of patient-specific organ doses for CT using deep-learning-based multi-organ segmentation and GPU-accelerated Monte Carlo dose computing. Med Phys 47(6):2526–2536
Kearney V, Chan JW, Haaf S, Descovich M, Solberg TD (2018) DoseNet: a volumetric dose prediction algorithm using 3D fully-convolutional neural networks. Phys Med Biol 63(23):235022
European Commission (2014) Council Directive 2013/59/Euratom of 5 December 2013. Off J Eur Union. https://doi.org/10.3000/19770677.L_2013.124.eng
Funding
This study has received funding from the research project “Patient dosimetry in computed tomography, interventional radiology and mammography” (KA10697).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Prof. John Damilakis.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was not required for this study.
Ethical approval
Institutional review board approval was obtained.
Methodology
• Retrospective
• Experimental
• Performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(RAR 927 kb)
Rights and permissions
About this article
Cite this article
Tzanis, E., Damilakis, J. A novel methodology to train and deploy a machine learning model for personalized dose assessment in head CT. Eur Radiol 32, 6418–6426 (2022). https://doi.org/10.1007/s00330-022-08756-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-08756-w