Abstract
Objectives
To compare diagnostic performances of current guidelines for the diagnosis of HCC in LT candidates using gadoxetic acid–enhanced liver MRI (Gd-EOB-MRI).
Methods
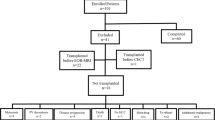
Eighty-one patients (119 HCCs and 35 non-HCCs) who underwent preoperative Gd-EOB-MRI and subsequent LT were included. Per-lesion imaging diagnoses of HCCs were made using four different guidelines (American Association for the Study of Liver Disease (AASLD), European Association for the Study of the Liver (EASL), Asian Pacific Association for the Study of the Liver (APASL), and Korean Liver Cancer Association-National Cancer Center (KLCA-NCC) guidelines, and patient allocation was determined according to Milan criteria (MC). Comparisons of per-lesion sensitivity, specificity, and accuracy of patient allocation between guidelines were performed using logistic regression with generalized estimating equations.
Results
For diagnosis of HCC, AASLD guideline showed highest specificity (97.4%), followed by EASL and KLCA-NCC guidelines (92.1% and 92.1%, p > 0.99 and = 0.15, respectively, in comparison to AASLD), while the specificity of APASL guideline was significantly lower than that of AASLD guideline (78.9% vs. 97.4%, p = 0.006). APASL and KLCA-NCC guidelines (75.9% and 65.6%) showed significantly higher sensitivities than AASLD/EASL guidelines (34.5% and 38.8%, respectively; all ps < 0.001). For organ allocation, KLCA-NCC guideline showed higher accuracy in selecting unsuitable candidates (with non-HCC malignancies or beyond MC HCCs) than EASL guideline (68.4% vs. 31.8%; p = 0.001).
Conclusion
For the diagnosis of HCCs using Gd-EOB-MRI, AASLD guideline provided the highest specificity, followed by EASL, KLCA-NCC, and APASL guidelines with statistically significant difference with only APASL guideline. KLCA-NCC guideline provided the most accurate selection of unsuitable LT candidates.
Key Points
• AASLD/LI-RADS showed the highest specificity, followed by EASL and KLCA-NCC guidelines.
• APASL and KLCA-NCC guidelines allowed more sensitive diagnoses of HCCs.
• KLCA-NCC more accurately classified patients not appropriate transplantation candidates than EASL.




Similar content being viewed by others
Abbreviations
- AASLD:
-
American Association for the Study of Liver Disease
- APASL:
-
Asian-Pacific Association for the Study of the Liver
- cHCC-CCAs:
-
Combined hepatocellular and cholangiocarcinomas
- EASL:
-
European Association for the Study of the Liver
- GEE:
-
Generalized estimating equation
- HCC:
-
Hepatocellular carcinoma
- KLCA-NCC:
-
Korean Liver Cancer Association-National Cancer Center
- LI-RADS:
-
Liver Imaging Reporting and Data System
- LT:
-
Liver transplantation
- MC:
-
Milan criteria
- MELD:
-
Model for end-stage liver disease
- MRI:
-
Magnetic resonance imaging
- OPTN:
-
Organ Procurement and Transplantation Network
- SD:
-
Standard deviation
- TACE:
-
Transarterial chemoembolization
- UNOS:
-
United Network for Organ Sharing
References
Villanueva A (2019) Hepatocellular carcinoma. N Engl J Med 380:1450–1462
Clavien PA, Lesurtel M, Bossuyt PM, Gores GJ, Langer B, Perrier A (2012) Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol 13:e11–e22
Wald C, Russo MW, Heimbach JK, Hussain HK, Pomfret EA, Bruix J (2013) New OPTN/UNOS policy for liver transplant allocation: standardization of liver imaging, diagnosis, classification, and reporting of hepatocellular carcinoma. Radiology 266:376–382
Fowler KJ, Sirlin CB (2019) Is it time to expand the definition of washout appearance in LI-RADS? Radiology 291:658–659
Kim TH, Kim SY, Tang A, Lee JM (2019) Comparison of international guidelines for noninvasive diagnosis of hepatocellular carcinoma: 2018 update. Clin Mol Hepatol 25:245–263
Omata M, Cheng AL, Kokudo N et al (2017) Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int 11:317–370
Marrero JA, Kulik LM, Sirlin CB et al (2018) Diagnosis, staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 68:723–750
Heimbach JK, Kulik LM, Finn RS et al (2018) AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 67:358–380
European Association for the Study of the Liver (2018) EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 69:182–236
Korean Liver Cancer Association; National Cancer Center (2019) 2018 Korean Liver Cancer Association-National Cancer Center Korea Practice Guidelines for the Management of Hepatocellular Carcinoma. Gut Liver 13:227–299
Kim TH, Yoon JH, Lee JM (2019) Emerging role of Hepatobiliary magnetic resonance contrast media and contrast-enhanced ultrasound for noninvasive diagnosis of hepatocellular carcinoma: emphasis on recent updates in major guidelines. Korean J Radiol 20:863–879
Lee YJ, Lee JM, Lee JS et al (2015) Hepatocellular carcinoma: diagnostic performance of multidetector CT and MR imaging-a systematic review and meta-analysis. Radiology 275:97–109
Edmondson HA, Steiner PE (1954) Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer 7:462–503
Chernyak V, Fowler KJ, Kamaya A et al (2018) Liver imaging reporting and data system (LI-RADS) version 2018: imaging of hepatocellular carcinoma in at-risk patients. Radiology 289:816–830
Ren AH, Zhao PF, Yang DW, Du JB, Wang ZC, Yang ZH (2019) Diagnostic performance of MR for hepatocellular carcinoma based on LI-RADS v2018, compared with v2017. J Magn Reson Imaging 50:746–755
Lee SM, Lee JM, Ahn SJ, Kang HJ, Yang HK, Yoon JH (2019) LI-RADS version 2017 versus version 2018: diagnosis of hepatocellular carcinoma on gadoxetate disodium-enhanced MRI. Radiology 292:655–663
Fraum TJ, Tsai R, Rohe E et al (2018) Differentiation of hepatocellular carcinoma from other hepatic malignancies in patients at risk: diagnostic performance of the liver imaging reporting and data system version 2014. Radiology 286:158–172
Ludwig DR, Fraum TJ, Cannella R et al (2019) Hepatocellular carcinoma (HCC) versus non-HCC: accuracy and reliability of liver imaging reporting and data system v2018. Abdom Radiol (NY) 44:2116–2132
Kulik L, Heimbach JK, Zaiem F et al (2018) Therapies for patients with hepatocellular carcinoma awaiting liver transplantation: a systematic review and meta-analysis. Hepatology 67:381–400
Mehta N, Sarkar M, Dodge JL, Fidelman N, Roberts JP, Yao FY (2016) Intention to treat outcome of T1 hepatocellular carcinoma with the “wait and not ablate” approach until meeting T2 criteria for liver transplant listing. Liver Transpl 22:178–187
Bruix J, Reig M, Sherman M (2016) Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology 150:835–853
Harper AM, Edwards E, Washburn WK, Heimbach J (2016) An early look at the organ procurement and transplantation network explant pathology form data. Liver Transpl 22:757–764
Freeman RB, Mithoefer A, Ruthazer R et al (2006) Optimizing staging for hepatocellular carcinoma before liver transplantation: a retrospective analysis of the UNOS/OPTN database. Liver Transpl 12:1504–1511
Park JW, Chen M, Colombo M et al (2015) Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE study. Liver Int 35:2155–2166
Tang A, Cruite I, Mitchell DG, Sirlin CB (2018) Hepatocellular carcinoma imaging systems: why they exist, how they have evolved, and how they differ. Abdom Radiol (NY) 43:3–12
Lee DH, Lee JM, Baek JH, Shin CI, Han JK, Choi BI (2015) Diagnostic performance of gadoxetic acid-enhanced liver MR imaging in the detection of HCCs and allocation of transplant recipients on the basis of the Milan criteria and UNOS guidelines: correlation with histopathologic findings. Radiology 274:149–160
Kim DH, Choi SH, Kim SY, Kim MJ, Lee SS, Byun JH (2019) Gadoxetic acid-enhanced MRI of hepatocellular carcinoma: value of washout in transitional and hepatobiliary phases. Radiology 291:651–657
Joo I, Lee JM, Lee DH, Jeon JH, Han JK (2019) Retrospective validation of a new diagnostic criterion for hepatocellular carcinoma on gadoxetic acid-enhanced MRI: can hypointensity on the hepatobiliary phase be used as an alternative to washout with the aid of ancillary features? Eur Radiol 29:1724–1732
Kierans AS, Makkar J, Guniganti P et al (2019) Validation of liver imaging reporting and data system 2017 (LI-RADS) criteria for imaging diagnosis of hepatocellular carcinoma. J Magn Reson Imaging 49:e205–e215
Jeon SK, Joo I, Lee DH et al (2019) Combined hepatocellular cholangiocarcinoma: LI-RADS v2017 categorisation for differential diagnosis and prognostication on gadoxetic acid-enhanced MR imaging. Eur Radiol 29:373–382
Acknowledgements
The authors thank Chris Woo for his editorial assistance.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Jeong Min Lee.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• diagnostic or prognostic study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 37 kb).
Rights and permissions
About this article
Cite this article
Jeon, S.K., Lee, J.M., Joo, I. et al. Comparison of guidelines for diagnosis of hepatocellular carcinoma using gadoxetic acid–enhanced MRI in transplantation candidates. Eur Radiol 30, 4762–4771 (2020). https://doi.org/10.1007/s00330-020-06881-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-06881-y