Abstract
Introduction
It has been reported that Th2 cytokines down-regulate antitumor immunity, while activation of type 1 T cell responses promotes antitumor immunity. However, detailed information on the immunological background of patients with HCC is still unknown. The objectives of this study were to evaluate the Th1/Th2 balance and to investigate the relation between carcinogenesis and host immunity in patients with chronic hepatitis C, HCV-related liver cirrhosis (LC), or HCC.
Patients/methods
The study population was 117 patients who had chronic inflammation due to HCV infection diagnosed from pathological examination of liver biopsy specimens, including 32 patients who had HCV-related LC with HCC. Apart from the patients with HCC, they were divided into the four subgroups based on the fibrosis score of Desment (stages 1–4). Blood samples were collected in the early morning before treatment. Flow cytometry was used to assess cytoplasmic IFN-gamma and IL-4 expression by peripheral blood CD4+ T cells, and the percentage of IFN-gamma+ and IL4− T cells (Th1) or IFN-gamma- and IL4+ T cells (Th2) was calculated before the start of each therapy.
Results
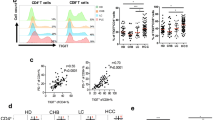
There were 20 patients in F1, 25 patients in F2, 19 patients in F3, 21 patients in F4, and 32 patients with HCC. In the F4 and HCC groups, Th1 cells tended to increase depending on the extent of fibrosis, although there were no significant differences between these groups and the other groups. In the HCC group, Th2 cells showed a significantly higher percentage than in the F1 or F3 groups.
Conclusions
These results suggest that Th1 dominance is lost due to an increase of Th2 cells in HCC patients and that carcinogenesis might occur in patients with chronic HCV infection and increased level of Th2 cells.









Similar content being viewed by others
References
Wada Y, Nakashima O, Kutani R, Yamamoto O, Kojiro M (1998) Clinicopathological study on hepatocellular carcinoma with lymphocytic infiltration. Hepatology 27:407–414
Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montaltof F, Ammatuna M, Morabito A, Gennari L (1996) Liver transplantation for the treatment of small hepatocellular carcinoma in patients with cirrhosis. N Engl J Med 334:693–700
Schirren CA, Jung M, Worzfeld T, Mamin M, Baretton GB, Gruener NH, Gerlach JT, Diepolder HM, Zachoval R, Pape GR (2000) Cytokine profile of liver- and blood-derived nonspecific T cells after liver transplantation. Liver Transplant 6:222–228
Marinos G, Rossol S, Carucci O, Wong PY, Danaldson P, Hussain MJ, Vergani D, Portmann BC, Williams R, Naoumov NV (2000) Immunopathogenesis of hepatitis B virus recurrence after liver transplantation. Transplantation 69:559–568
Mosmann TR, Coffman RL (1989) Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol 7:145–173
O’Garra A (1998) Cytokines induce the development of functionally heterogenous T helper cell subsets. Immunity 8:275–283
Vincent MS, Gumperz JE, Brenner MB (2003) Understanding the function of CD1d-restricted T cells. Nat Immunol 6:517–523
Nakajima H, Mizushima N, Kanai K (1987) Relationship between natural killer activity and development of hepatocellular carcinoma in patients with cirrhosis of the liver. Jpn J Clin Oncol 17:327–332
Kobayashi M, Kobayashi H, Polland RB, Suzuki F (1998) A pathogenic role of Th2 cells and their cytokine products on the pulmonary metastasis of murine B16 melanoma. J Immunol 160:5869–5873
Zitvogel L, Mayordomo JI, Tjandrawan T, Deleo AB, Clarke MR, Lotze MT, Storkus WJ (1996) Therapy of murine tumors with tumor peptide-pulsed dendritic cells: dependence on T cells, B7 costimulation, and T helper cell 1-associated cytokines. J Exp Med 183:87–97
Tsung K, Meko JB, Peplinski GR, Norton JA (1997) IL-12 induces T helper 1-directed antitumor response. J Immunol 158:3359–3365
Weiner GJ, Liu HM, Wooldridge JE, Dahle CE, Krieg AM (1997) Immunostimulatory oligodeoxynucleotides containing the CpG motif are effective as immune adjuvants in tumor antigen immunization. Proc Natl Acad Sci USA 94:10833–10837
Hu H-M, Urba WJ, Fox BA (1998) Gene-modified tumor vaccine with therapeutic potential shifts tumor-specific T cell response from a type 2 to a type 1 cytokine profile. J Immunol 161:3033
Shinohara M, Ishii K, Takamura N, Takamura N (2003) Long-term changes of peripheral blood CD4-positive T cell subsets (Th1, Th2) in chronic hepatitis C patients with a sustained response no response to IFN. Hepatol Res 27:260–265
Jung T, Schauer U, Heusser C, Neumann C, Rieger C (1993) Detection of intracellular cytokines by flow cytometry. J Immunol Methods 159:197–207
Kaser T, Enrich B, Ludwiczek O, Vogel W, Tilg H (1999) Interferon-alpha (INF-alpha) enhances cytotoxicity in healthy volunteers and chronic hepatitis C infection mainly by the perforin pathway. Clin Exp Immunol 118:71–77
Fang SH, Hwang LH, Chen DS, Chiang BL (2000) Ribavirin enhancement of hepatitis C virus core antigen-specific type 1 T helper cell response correlates with the increased IL-12 level. J Hepatol 33:791–798
Takamura N, Ishii K, Shinohara M, Matsumaru K, Kawafune T, Sumino Y (2002) Analysis of peripheral blood CD4 positive T cells using intracellular staining method in paients with chronic hepatitis C. J Med Soc Toho 49(1):45–52
Cao Z, Chen X, Wu Z (2007) Effect of splenectomy in patients with cirrhosis undergoing hepatic resection for hepatocellular carcinoma. World J Gastroenterol 13(2):280–284
Sakaguchi E, Kayano K, Segawa M, Okamoto M, Sakaida I, Okita K (2002) Th1 down-regulation at the single-lymphocyte level in HCV-related liver cirrhosis and the effect of TGF-β on Th1 response: possible implication for the development of hepatoma. Hepatol Res 24:282–289
Kojo S, Adachi Y, Keino H, Taniguchi M, Sumida T (2001) Dysfunction of T cell receptor Vα24Jα18+, vβ??+(double-negative regulatory natural killer T cells in autoimmune disease. Arthritis Rheum 44:1127–1136
Kita H, Imawari M, Gershwin ME (2004) Cellular immune response in primary bilialy cirrhosis. Hepatol Res 28:12–17
Takeda K, Hayakawa Y, Van Kaer L, Matsuda H, Yagita H, Okumura K (2000) Critical contribution of liver natural killer T cells to a murine model of hepatitis. Proc Natl Acad Sci USA 97:5498–5503
Kawano T, Nakayama T, Kamada N, Kaneko Y, Harada M, Ogura(N, Akutsu Y, Motohashi S, Iizawa T, Endo H, Fujisawa T, Shinkai H, Taniguchi M (1999) Antitumor cytotoxicity mediated by ligand-activated human Vα24 NKT cells. Cancer Res 59:5102–5105
Doherty DG, Norris S, Madrigal-Estebas L, McEntee G, Traynor O, Hegarty JE, O’Farrelly C (1999) The human liver contains multiple population of NK cells, T cells, and CD3 + CD56+ natural T cell with distinct cytotoxic activities and Th1, Th2, and Th0 cytokine secretion patterns. J Immunol 163:2314–2321
Tartter PA, Steinberg B, Barron DM, Martinelli G (1987) The prognostic significance of natural killer cytotoxicity in patients with colorectal cancer. Arch Surg 122:1264–1268
Exley MA, He Q, Cheng O, Wang RJ, Cheney CP, Balk SP, Kuziel MJ (2002) Compartmentalization of Th1-like noninvariant CD1d-reactive T cells in hepatitis C virus-infected liver. J Immunol 168:1519–1523
Poccia F, Agrati C (2003) Intrahepatic natural immunity and HCV immunopathogenesis. Cell Death Differ 10:S9–S12
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matsui, T., Nagai, H., Sumino, Y. et al. Relationship of peripheral blood CD4-positive T cells to carcinogenesis in patients with HCV-related chronic hepatitis and liver cirrhosis. Cancer Chemother Pharmacol 62, 401–406 (2008). https://doi.org/10.1007/s00280-007-0618-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-007-0618-1