Abstract
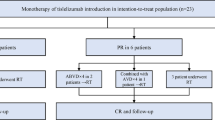
Immune checkpoint inhibitors (ICI) have demonstrated high therapeutic efficacy in relapsed or refractory classical Hodgkin lymphoma (r/r cHL). Nevertheless, despite the accumulated data, the question of the ICI therapy duration and efficacy of nivolumab retreatment remains unresolved. In this retrospective study, in a cohort of 23 adult patients with r/r cHL who discontinued nivolumab in complete response (CR), the possibility of durable remission achievement (2-year PFS was 55.1%) was demonstrated. Retreatment with nivolumab has demonstrated efficacy with high overall response rate (ORR) and CR (67% and 33.3% respectively). At the final analysis, all patients were alive with median PFS of 16.5 months. Grade 3–4 adverse events (AEs) were reported in 36% of patients, and there was no deterioration in terms of nivolumab retreatment–associated complications.




Similar content being viewed by others
Data Availability
Not applicable
References
Armand P, Engert A, Younes A, Fanale M, Santoro A, Zinzani PL, Timmerman JM, Collins GP, Ramchandren R, Cohen JB, de Boer JP, Kuruvilla J, Savage KJ, Trneny M, Shipp MA, Kato K, Sumbul A, Farsaci B, Ansell SM (2018) Nivolumab for relapsed/refractory classic Hodgkin lymphoma after failure of autologous hematopoietic cell transplantation: extended follow-up of the multicohort single-arm phase II CheckMate 205 trial. J Clin Oncol 36(14):1428–1439. https://doi.org/10.1200/JCO.2017.76.0793
Chen R, Zinzani PL, Fanale MA, Armand P, Johnson NA, Brice P, Radford J, Ribrag V, Molin D, Vassilakopoulos TP, Tomita A, von Tresckow B, Shipp MA, Zhang Y, Ricart AD, Balakumaran A, Moskowitz CH, for the KEYNOTE-087 (2017) KEYNOTE-087. Phase II study of the efficacy and safety of pembrolizumab for relapsed/refractory classic Hodgkin lymphoma. J Clin Oncol 35(19):2125–2132. https://doi.org/10.1200/JCO.2016.72.1316
Gauci M, Lanoy E, Champiat S, Caramella C et al (2019) Long-term survival in patients responding to anti-PD-1/PD-L1 therapy and disease outcome upon treatment discontinuation. Clinical Cancer Research 25(3):946–956. https://doi.org/10.1158/1078-0432.ccr-18-0793
Tachihara M, Negoro S, Inoue T, Tamiya M, Akazawa Y, Uenami T, Urata Y, Hattori Y, Hata A, Katakami N, Yokota S (2018) Efficacy of anti-PD-1/PD-L1 antibodies after discontinuation due to adverse events in non-small cell lung cancer patients (HANSHIN 0316). BMC Cancer 18:946. https://doi.org/10.1186/s12885-018-4819-2
Jansen YJL, Rozeman EA, Mason R, Goldinger SM, Geukes Foppen MH, Hoejberg L, Schmidt H, van Thienen JV, Haanen JBAG, Tiainen L, Svane IM, Mäkelä S, Seremet T, Arance A, Dummer R, Bastholt L, Nyakas M, Straume O, Menzies AM, Long GV, Atkinson V, Blank CU, Neyns B (2019) Discontinuation of anti-PD-antibody therapy in the absence of disease progression or treatment limiting toxicity: clinical outcomes in advanced melanoma. Annals of Oncology 30(7):1154–1161. https://doi.org/10.1093/annonc/mdz110
Myint ZW, Arays R, Raajasekar AKA, Wang P (2018) Long-term outcomes in patients after discontinuation of immune checkpoint inhibitors. J Clin Res Med 1(3):1–4. https://doi.org/10.1200/JCO.2018.36.15_suppl.e15086
Atkinson VG, Ladwa R (2016) Complete responders to anti-PD1 antibodies. What happens when we stop? Ann Oncol 27(6):1116P. https://doi.org/10.1093/annonc/mdw379.11
Myint Z, Arays R, Raajasekar AKA, Huang B, Chen Q, Wang P (2018) Long-term outcomes in patients after discontinuation of PD1/PDL1 inhibitors. J Clin Oncol 36:e15086. https://doi.org/10.1200/JCO.2018.36.15_suppl.e15086
Spigel DR, et al (2017) CHECKMATE153: Randomized results of continuous vs 1-year fixed-duration nivolumab in patients with advanced non-small cell lung cancer. Ann Oncol 28:5. Abstract 1297O. https://www.primeoncology.org/app/uploads/prime_activities/42584/VPS_Madrid_1297O_Lung_Spigel.pdf
Manson G, Herbaux C, Brice P, Bouabdallah K, Stamatoullas A, Schiano JM, Ghesquieres H, Dercle L, Houot R, Lymphoma Study Association (2018) Prolonged remissions after anti-PD-1 discontinuation in patients with Hodgkin lymphoma. Blood 131:2856–2859. https://doi.org/10.1182/blood-2018-03-841262
Lepik K, Fedorova L, Mikhailova N et al (2019) Cessation of immune checkpoint inhibitors therapy in r/r Hodgkin lymphoma: should we consider HSCT? 45th Annual Meeting of the European Society for Blood and Marrow Transplantation, Abstract collection. Poster number A232
Goodman A (2017) Retreatment with checkpoint inhibitors may be feasible for some patients with NSCLC. The ASCO post. https://www.ascopost.com/issues/july-10-2017/retreatment-with-checkpoint-inhibitors-may-be-feasible-for-some-patients-with-nsclc. Accessed 10 June 2017
Santini FC, Rizvi H, Wilkins O, van Voorthuysen M, Panora E, Halpenny D, Kris MG, Rudin CM, Chaft JE, Hellmann MD (2017) Safety of retreatment with immunotherapy after immune-related toxicity in patients with lung cancers treated with anti-PD(L)-1 therapy. J Clin Oncol 35(15):9012. https://doi.org/10.1200/JCO.2017.35.15_suppl.9012
Fujita K, Uchida N, Yamamoto Y, et al (2019) Retreatment with anti-PD-L1 antibody in advanced non-small cell lung cancer previously treated with anti-PD-1 antibodies. Anticancer Research 39(7):3917–3921. 10.21873/anticanres.13543
Alaiwi SA, Martini DJ, Xie W et al (2019) Safety and efficacy of restarting immune checkpoint inhibitors (ICI) after immune-related adverse events (irAEs) in metastatic renal cell carcinoma (mRCC). J Clin Oncol 37(7):652–652. https://doi.org/10.1200/JCO.2019.37.7_suppl.652
Pollack MH, Betof A, Dearden H, Rapazzo K, Valentine I, Brohl AS, Ancell KK, Long GV, Menzies AM, Eroglu Z, Johnson DB, Shoushtari AN (2018) Safety of resuming anti-PD-1 in patients with immune-related adverse events (irAEs) during combined anti-CTLA-4 and anti-PD1 in metastatic melanoma. Ann Oncol 29:250–255. https://doi.org/10.1093/annonc/mdx642
Lebbé C, Weber JS, Maio M, Neyns B, Harmankaya K, Hamid O, O'Day SJ, Konto C, Cykowski L, McHenry MB, Wolchok JD (2014) Survival follow-up and ipilimumab retreatment of patients with advanced melanoma who received ipilimumab in prior phase II studies. Ann Oncol 25:2277–2284. https://doi.org/10.1093/annonc/mdu441
Chiarion-Sileni V, Pigozzo J, Ascierto PA, Simeone E, Maio M, Calabrò L, Marchetti P, de Galitiis F, Testori A, Ferrucci PF, Queirolo P, Spagnolo F, Quaglino P, Carnevale Schianca F, Mandalà M, di Guardo L, del Vecchio M (2014) Ipilimumab retreatment in patients with pretreated advanced melanoma: the expanded access programme in Italy. Br J Cancer 110:1721–1726. https://doi.org/10.1038/bjc.2014.126
Robert C, Schadendorf D, Messina M, Hodi FS, O'Day S, MDX010-20 investigators (2013) Efficacy and safety of retreatment with ipilimumab in patients with pretreated advanced melanoma who progressed after initially achieving disease control. Clin Cancer Res 19:2232–2239. https://doi.org/10.1158/1078-0432.CCR-12-3080
Maiko N, Nakaya A, Kurata T, et al (2018) Immune checkpoint inhibitor re-challenge in patients with advanced non-small cell lung cancer. Oncotarget 9(64):32298–32304. 10.18632/oncotarget.25949
Pellegrini C, Casadei B, Cellini C, Argnani L, Cavo M, Zinzani PL (2018) Sequential double bridging to transplant with diversified anti-PD1 monoclonal antibodies retreatment in relapsed Hodgkin lymphoma: a case report. J Cancer Sci Ther 10(6):149. https://doi.org/10.4172/1948-5956.1000534
Chen R, Zinzani PL, Lee HJ, Armand P, Johnson NA, Brice P, Radford J, Ribrag V, Molin D, Vassilakopoulos TP, Tomita A, von Tresckow B, Shipp MA, Lin J, Kim E, Nahar A, Balakumaran A, Moskowitz CH (2019) Pembrolizumab in relapsed or refractory Hodgkin lymphoma: two-year follow-up of KEYNOTE-087. Blood. 134:1144–1153. https://doi.org/10.1182/blood.2019000324
Lepik KV, Mikhailova NB, Moiseev IS, et al (2019) Nivolumab for the treatment of relapsed and refractory classical Hodgkin lymphoma after ASCT and in ASCTnaïve patients. Leukemia & Lymphoma 1–4. https://doi.org/10.1080/10428194.2019.1573368
Kozlov AV, Kazantzev IV, Iukhta TV, et al (2019) Nivolumab in pediatric Hodgkin’s lymphoma. CTT. 10.18620/ctt-1866-8836-2019-8-4-41-48
Lepik KV, Fedorova LV, Kondakova EV et al (2020) A phase 2 study of nivolumab using a fixed dose of 40 mg (Nivo40) in patients with relapsed/refractory Hodgkin lymphoma. Hemasphere 4(5):480. 2020 https://doi.org/10.1097/HS9.0000000000000480
Shi Y, Su H, Song Y, Jiang W, Sun X, Qian W, Zhang W, Gao Y, Jin Z, Zhou J, Jin C, Zou L, Qiu L, Li W, Yang J, Hou M, Zeng S, Zhang Q, Hu J, Zhou H, Xiong Y, Liu P (2019) Safety and activity of sintilimab in patients with relapsed or refractory classical Hodgkin lymphoma (Orient-1): a multicentre, single-arm, phase 2 trial. Lancet Haematol 6:e12–e19. https://doi.org/10.1016/S2352-3026(18)30192-3
Song Y, Gao Q, Zhang H, Fan L, Zhou J, Zou D, Li W, Yang H, Liu T, Wang Q, Lv F, Yang Y, Guo H, Yang L, Elstrom R, Huang J, Novotny W, Wei V, Zhu J (2018) Tislelizumab (Bgb-A317) for relapsed/refractory classical Hodgkin lymphoma: preliminary efficacy and safety results from a phase 2 study. Blood 132:682. https://doi.org/10.1182/blood-2018-99-117848
Nie J, Wang C, Liu Y, Yang Q, Mei Q, Dong L, Li X, Liu J, Ku W, Zhang Y, Chen M, An X, Shi L, Brock MV, Bai J, Han W (2019) Addition of low-dose decitabine to anti-Pd-1 antibody camrelizumab in relapsed/refractory classical Hodgkin lymphoma. J Clin Oncol 37:1479–1489. https://doi.org/10.1200/JCO.18.02151
Chen R, Gibb AL, Collins GP, Popat R, el-Sharkawi D, Burton C, Lewis D, Miall FM, Forgie A, Compagnoni A, Andreola G, Brar S, Thall A, Woolfson A, Radford J (2017) Blockade of the PD-1 checkpoint with anti–PD-L1 antibody Avelumab is sufficient for clinical activity in relapsed/refractory classical Hodgkin lymphoma (cHL). Hematol Oncol 35:67. https://doi.org/10.1002/hon.2437_54
Hamadani M, Collins GP, Samaniego F, Spira AI, Davies A, Radford J, Caimi P, Menne T, Boni J, Cruz H, Feingold J, He S, Wuerthner J, Horwitz SM (2018) Phase 1 study of Adct-301 (Camidanlumab Tesirine), a novel pyrrolobenzodiazepine-based antibody drug conjugate, in relapsed/refractory classical Hodgkin lymphoma. Blood 132:928. https://doi.org/10.1182/blood-2018-99-118198
Rothe A, Sasse S, Topp MS, Eichenauer DA, Hummel H, Reiners KS, Dietlein M, Kuhnert G, Kessler J, Buerkle C, Ravic M, Knackmuss S, Marschner JP, Pogge von Strandmann E, Borchmann P, Engert A (2015) A phase 1 study of the bispecific anti-CD30/CD16A antibody construct Afm13 in patients with relapsed or refractory Hodgkin lymphoma. Blood 125:4024–4031. https://doi.org/10.1182/blood-2014-12-614636
Smith SM, Schöder H, Johnson JL, et al (2013) The Anti-CD80 primatized monoclonal antibody, galiximab, is well-tolerated but has limited activity in relapsed Hodgkin lymphoma: cancer and leukemia group B 50602 (Alliance) Leukemia & Lymphoma 54:1405–1410. 10.3109/10428194.2012.744453
Freedman AS, Kuruvilla J, Assouline SE et al (2010) Clinical activity of lucatumumab (Hcd122) in patients (Pts) with relapsed/refractory Hodgkin or non-Hodgkin lymphoma treated in a phase Ia/II clinical trial (NCT00670592). Blood 116:284. https://doi.org/10.1182/blood.V116.21.284.284
Lepik KV, Mikhailova NB, Kondakova EV, Zalyalov YR, Fedorova LV, Tsvetkova LA, Kotselyabina PV, Borzenkova ES, Babenko EV, Popova MO, Darskaya EI, Baykov VV, Moiseev IS, Afanasyev BV (2020) A study of safety and efficacy of nivolumab and bendamustine (NB) in patients with relapsed/refractory Hodgkin lymphoma after nivolumab monotherapy failure. HemaSphere 4:e401. https://doi.org/10.1097/HS9.0000000000000401
Acknowledgements
The authors thank Alexander Kulagin, Ilya Kazantzev, and Vladislav Evseev for assistance with manuscript preparation.
Author information
Authors and Affiliations
Contributions
Conception and design: Fedorova LV, Lepik KV, Mikhailova NB, Moiseev IS, Afanasyev BV
Provision of study materials or patients: Fedorova LV, Lepik KV, Mikhailova NB, Kondakova EV, Zalyalov YR, Kozlov AV, Babenko EV, Baykov VV
Collection and assembly of data: Fedorova LV, Lepik KV, Mikhailova NB, Kondakova EV, Zalyalov YR, Kozlov AV
Data analysis and interpretation: Fedorova LV, Lepik KV, Mikhailova NB, Kondakova EV, Moiseev IS, Afanasyev BV.
Manuscript writing: Fedorova LV, Lepik KV, Mikhailova NB
Final approval of manuscript: all authors
Accountable for all aspects of the work: Fedorova LV, Lepik KV, Mikhailova NB
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was performed in accordance with the 1964 Helsinki declaration and approved by the institutional review board. All enrolled patients gave written informed consent.
Consent for publication
Not applicable
Conflict of interest
The authors declare no competing interests.
Code availability
Not applicable
Materials availability
Not applicable
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fedorova, L.V., Lepik, K.V., Mikhailova, N.B. et al. Nivolumab discontinuation and retreatment in patients with relapsed or refractory Hodgkin lymphoma. Ann Hematol 100, 691–698 (2021). https://doi.org/10.1007/s00277-021-04429-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-021-04429-8