Abstract
Purpose
To use absolute pretreatment apparent diffusion coefficients (ADC) derived from diffusion-weighted MR imaging (DWI) to predict response to repetitive cTACE for unresectable liver metastases of colorectal carcinoma (CRLM) at 1 and 3 months after start of treatment.
Materials and Methods
Fifty-five metastases in 34 patients were examined with DWI prior to treatment and 1 month after initial cTACE. Treatment was performed in 4-week intervals. Response was evaluated at 1 and 3 months after start of therapy. Metastases showing a decrease of ≥30% in axial diameter were classified as responding lesions.
Results
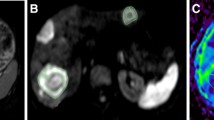
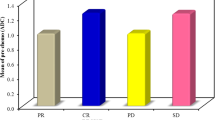
One month after initial cTACE, seven lesions showed early response. There was no significant difference in absolute pretreatment ADC values between responding and non-responding lesions (p = 0.94). Three months after initial cTACE, 17 metastases showed response. There was a significant difference (p = 0.021) between absolute pretreatment ADC values of lesions showing response (median 1.08 × 10−3 mm2/s) and no response (median 1.30 × 10−3 mm2/s). Pretreatment ADC showed fair diagnostic value to predict response (AUC 0.7). Lesions showing response at 3 months also revealed a significant increase in ADC between measurements before treatment and at one month after initial cTACE (p < 0.001). Applying an increase in ADC of 12.17%, response at 3 months after initial cTACE could be predicted with a sensitivity and specificity of 77 and 74%, respectively (AUC 0.817). Furthermore, there was a strong and significant correlation (r = 0.651, p < 0.001) between percentage change in size after third cTACE and percentage change in ADC.
Conclusion
In patients with CRLM, ADC measurements are potential biomarkers for assessing response to cTACE.





Similar content being viewed by others
References
McGuire S. World Cancer Report 2014. Geneva, Switzerland: World Health Organization, international agency for research on cancer, WHO Press, 2015. Adv Nutr. 2016;7(2):418–9. doi:10.3945/an.116.012211.
Jonker DJ, Maroun JA, Kocha W. Survival benefit of chemotherapy in metastatic colorectal cancer: a meta-analysis of randomized controlled trials. Br J Cancer. 2000;82(11):1789–94. doi:10.1054/bjoc.1999.1254.
Gruber-Rouh T, Naguib NN, Eichler K, Ackermann H, Zangos S, Trojan J, et al. Transarterial chemoembolization of unresectable systemic chemotherapy-refractory liver metastases from colorectal cancer: long-term results over a 10-year period. Int J Cancer. 2014;134(5):1225–31. doi:10.1002/ijc.28443.
Koh D-M, Scurr E, Collins D, Kanber B, Norman A, Leach MO, et al. Predicting response of colorectal hepatic metastasis: value of pretreatment apparent diffusion coefficients. Am J Roentgenol. 2007;188(4):1001–8. doi:10.2214/AJR.06.0601.
Bammer R. Basic principles of diffusion-weighted imaging. Eur J Radiol. 2003;45(3):169–84.
Kele PG, van der Jagt EJ. Diffusion weighted imaging in the liver. World J Gastroenterol. 2010;16(13):1567–76.
Koh DM, Collins DJ, Wallace T, Chau I, Riddell AM. Combining diffusion-weighted MRI with Gd-EOB-DTPA-enhanced MRI improves the detection of colorectal liver metastases. Br J Radiol. 1015;2012(85):980–9. doi:10.1259/bjr/91771639.
Qayyum A. Diffusion-weighted imaging in the abdomen and pelvis: concepts and applications. Radiogr A Rev Publ Radiol Soc North Am Inc. 2009;29(6):1797–810. doi:10.1148/rg.296095521.
Chapiro J, Wood LD, Lin M, Duran R, Cornish T, Lesage D, et al. Radiologic-pathologic analysis of contrast-enhanced and diffusion-weighted MR imaging in patients with HCC after TACE: diagnostic accuracy of 3D quantitative image analysis. Radiology. 2014;273(3):746–58. doi:10.1148/radiol.14140033.
Chung JC, Naik NK, Lewandowski RJ, Deng J, Mulcahy MF, Kulik LM, et al. Diffusion-weighted magnetic resonance imaging to predict response of hepatocellular carcinoma to chemoembolization. World J Gastroenterol. 2010;16(25):3161–7.
Venturini M, Pilla L, Agostini G, Cappio S, Losio C, Orsi M, et al. Transarterial chemoembolization with drug-eluting beads preloaded with irinotecan as a first-line approach in uveal melanoma liver metastases: tumor response and predictive value of diffusion-weighted MR imaging in five patients. J Vasc Interv Radiol. 2012;23(7):937–41. doi:10.1016/j.jvir.2012.04.027.
Schmeel FC, Simon B, Sabet A, Luetkens JA, Traber F, Schmeel LC, et al. Diffusion-weighted magnetic resonance imaging predicts survival in patients with liver-predominant metastatic colorectal cancer shortly after selective internal radiation therapy. Eur Radiol. 2016;. doi:10.1007/s00330-016-4430-3.
Buijs M, Kamel IR, Vossen JA, Georgiades CS, Hong K, Geschwind JF. Assessment of metastatic breast cancer response to chemoembolization with contrast agent enhanced and diffusion-weighted MR imaging. J Vasc Interv Radiol. 2007;18(8):957–63. doi:10.1016/j.jvir.2007.04.025.
Seldinger SI. Catheter replacement of the needle in percutaneous arteriography: a new technique. Acta Radiol. 1953;39(5):368–76. doi:10.3109/00016925309136722.
Vogl TJ, Muller PK, Mack MG, Straub R, Engelmann K, Neuhaus P. Liver metastases: interventional therapeutic techniques and results, state of the art. Eur Radiol. 1999;9(4):675–84. doi:10.1007/s003300050732.
Gruber-Rouh T, Marko C, Thalhammer A, Nour-Eldin NE, Langenbach M, Beeres M, et al. Current strategies in interventional oncology of colorectal liver metastases. Br J Radiol. 2016;89:20151060. doi:10.1259/bjr.20151060.
El Kady RM, Choudhary AK, Tappouni R. Accuracy of apparent diffusion coefficient value measurement on pacs workstation: a comparative analysis. Am J Roentgenol. 2011;196(3):W280–4. doi:10.2214/AJR.10.4706.
Deckers F, De Foer B, Van Mieghem F, Botelberge T, Weytjens R, Padhani A, et al. Apparent diffusion coefficient measurements as very early predictive markers of response to chemotherapy in hepatic metastasis: a preliminary investigation of reproducibility and diagnostic value. J Magn Reson Imaging. 2014;40(2):448–56. doi:10.1002/jmri.24359.
Taouli B, Koh D-M. Diffusion-weighted MR imaging of the liver. Radiology. 2010;254(1):47–66. doi:10.1148/radiol.09090021.
Vogl TJ, Gruber T, Balzer JO, Eichler K, Hammerstingl R, Zangos S. Repeated transarterial chemoembolization in the treatment of liver metastases of colorectal cancer: prospective study. Radiology. 2009;250(1):281–9. doi:10.1148/radiol.2501080295.
Evans JD. Straightforward statistics for the behavioral sciences. Pacific Grove: Brooks/Cole Publ. Co: An International Thomson Publ. Co; 1996.
Patterson DM, Padhani AR, Collins DJ. Technology insight: water diffusion MRI–a potential new biomarker of response to cancer therapy. Nat Clin Pract Oncol. 2008;5(4):220–33. doi:10.1038/ncponc1073.
Kokabi N, Ludwig JM, Camacho JC, Xing M, Mittal PK, Kim HS. Baseline and early MR apparent diffusion coefficient quantification as a predictor of response of unresectable hepatocellular carcinoma to doxorubicin drug-eluting bead chemoembolization. J Vasc Interv Radiol. 2015;26(12):1777–86. doi:10.1016/j.jvir.2015.08.023.
Cui Y, Zhang X-P, Sun Y-S, Tang L, Shen L. Apparent diffusion coefficient: potential imaging biomarker for prediction and early detection of response to chemotherapy in hepatic metastases. Radiology. 2008;248(3):894–900. doi:10.1148/radiol.2483071407.
Thoeny HC, De Keyzer F, Chen F, Vandecaveye V, Verbeken EK, Ahmed B, et al. Diffusion-weighted magnetic resonance imaging allows noninvasive in vivo monitoring of the effects of combretastatin a-4 phosphate after repeated administration. Neoplasia (New York, NY). 2005;7(8):779–87.
Harrison L, Blackwell K. Hypoxia and anemia: factors in decreased sensitivity to radiation therapy and chemotherapy? Oncologist. 2004;9(Suppl 5):31–40. doi:10.1634/theoncologist.9-90005-31.
Mannelli L, Kim S, Hajdu CH, Babb JS, Taouli B. Serial diffusion-weighted MRI in patients with hepatocellular carcinoma: prediction and assessment of response to transarterial chemoembolization. Prelim Exp Eur J Radiol. 2013;82(4):577–82. doi:10.1016/j.ejrad.2012.11.026.
Padhani AR, Liu G, Koh DM, Chenevert TL, Thoeny HC, Takahara T, et al. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia (New York, NY). 2009;11(2):102–25.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Lahrsow, M., Albrecht, M.H., Bickford, M.W. et al. Predicting Treatment Response of Colorectal Cancer Liver Metastases to Conventional Lipiodol-Based Transarterial Chemoembolization Using Diffusion-Weighted MR Imaging: Value of Pretreatment Apparent Diffusion Coefficients (ADC) and ADC Changes Under Therapy. Cardiovasc Intervent Radiol 40, 852–859 (2017). https://doi.org/10.1007/s00270-017-1634-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-017-1634-0