Abstract
Purpose
18F-Fluorodeoxyglucose positron emission tomography (FDG PET) may underestimate viable tumour tissue in patients with gastrointestinal stromal tumours (GIST) treated with molecular targeted agents. The aim of the present study was to investigate the value of parametric images generated after dynamic data acquisition for the detection of active liver metastases.
Methods
The analysis included 65 dynamic FDG PET studies in 34 patients with liver metastases from GIST who were treated with imatinib or sunitinib. Parametric images of intercept and slope were calculated by dedicated software using a voxel-based linear regression of time-activity data. Intercept images represent the tracer’s distribution volume and the slope its overall metabolic turnover. All images were assessed visually and semi-quantitatively. Liver disease status was established 12 months after each PET study. Dichotomous variables of visual interpretation and various quantitative parameters were entered in a statistical model of linear discriminant analysis.
Results
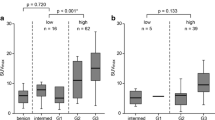
Visual analysis of slope images was more sensitive than the standard 1-h FDG uptake evaluation (70.6 vs 51.0%, p = 0.016) in detecting cases with liver disease progression (n = 51). Specificity did not differ. Combination of all variables in the discriminant analysis model correctly classified 87.7% of cases as progressive or non-progressive disease. Sensitivity was raised to 88.2%.
Conclusion
Parametric images of intercept and slope add a new dimension to the interpretation of FDG PET studies, by isolating visually and quantifying the perfusion and phosphorylation-dependent part of tracer uptake. In treated GIST patients, integration of this information with the 1-h uptake data achieves better characterization of hepatic lesions with respect to disease activity.





Similar content being viewed by others
References
Benjamin RS, Debiec-Rychter M, Le Cesne A, Sleijfer S, Demetri GD, Joensuu H, et al. Gastrointestinal stromal tumours II: medical oncology and tumour response assessment. Semin Oncol 2009;36:302–11.
Blanke CD, Demetri GD, von Mehren M, Heinrich MC, Eisenberg B, Fletcher JA, et al. Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol 2008;26:620–5.
Gold JS, van der Zwan SM, Gönen M, Maki RG, Singer S, Brennan MF, et al. Outcome of metastatic GIST in the era before tyrosine kinase inhibitors. Ann Surg Oncol 2007;14:134–42.
Duensing S, Duensing A. Targeted therapies of gastrointestinal stromal tumors (GIST)—the next frontiers. Biochem Pharmacol 2010;80:575–83.
Van den Abbeele AD. The lessons of GIST—PET and PET/CT: a new paradigm for imaging. Oncologist 2008;13 Suppl 2:8–13.
Prior JO, Montemurro M, Orcurto MV, Michielin O, Luthi F, Benhattar J, et al. Early prediction of response to sunitinib after imatinib failure by 18F-fluorodeoxyglucose positron emission tomography in patients with gastrointestinal stromal tumor. J Clin Oncol 2009;27:439–45.
Mabille M, Vanel D, Albiter M, Le Cesne A, Bonvalot S, Le Péchoux C, et al. Follow-up of hepatic and peritoneal metastases of gastrointestinal tumors (GIST) under imatinib therapy requires different criteria of radiological evaluation (size is not everything!!!). Eur J Radiol 2009;69:204–8.
Goerres GW, Stupp R, Barghouth G, Hany TF, Pestalozzi B, Dizendorf E, et al. The value of PET, CT and in-line PET/CT in patients with gastrointestinal stromal tumours: long-term outcome of treatment with imatinib mesylate. Eur J Nucl Med Mol Imaging 2005;32:153–62.
Stroobants S, Goeminne J, Seegers M, Dimitrijevic S, Dupont P, Nuyts J, et al. 18FDG-Positron emission tomography for the early prediction of response in advanced soft tissue sarcoma treated with imatinib mesylate (Glivec). Eur J Cancer 2003;39:2012–20.
Choi H, Charnsangavej C, de Castro Faria S, Tamm EP, Benjamin RS, Johnson MM, et al. CT evaluation of the response of gastrointestinal stromal tumors after imatinib mesylate treatment: a quantitative analysis correlated with FDG PET findings. AJR Am J Roentgenol 2004;183:1619–28.
Scaife CL, Hunt KK, Patel SR, Benjamin RS, Burgess MA, Chen LL, et al. Is there a role for surgery in patients with “unresectable” cKIT+ gastrointestinal stromal tumors treated with imatinib mesylate? Am J Surg 2003;186:665–9.
Sokoloff L. Mapping local functional activity by measurement of local cerebral glucose utilization in the central nervous system of animals and man. Harvey Lect 1983–1984;79:77–143.
Patlak CS, Blasberg RG. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Generalizations. J Cereb Blood Flow Metab 1985;5:584–90.
Burger C, Buck A. Requirements and implementation of a flexible kinetic modeling tool. J Nucl Med 1997;38:1818–23.
Schmidt KC, Turkheimer FE. Kinetic modeling in positron emission tomography. Q J Nucl Med 2002;46:70–85.
Dimitrakopoulou-Strauss A, Hoffmann M, Bergner R, Uppenkamp M, Eisenhut M, Pan L, et al. Prediction of short-term survival in patients with advanced nonsmall cell lung cancer following chemotherapy based on 2-deoxy-2-[F-18]fluoro-D-glucose-positron emission tomography: a feasibility study. Mol Imaging Biol 2007;9:308–17.
Dimitrakopoulou-Strauss A, Pan L, Strauss LG. Parametric imaging: a promising approach for the evaluation of dynamic PET-18F-FDG studies—the DKFZ experience. Hell J Nucl Med 2010;13:18–22.
Messa C, Choi Y, Hoh CK, Jacobs EL, Glaspy JA, Rege S, et al. Quantification of glucose utilization in liver metastases: parametric imaging of FDG uptake with PET. J Comput Assist Tomogr 1992;16:684–9.
Afifi A, Clark VA, May S. Computer-aided multivariate analysis. 4th ed. Boca Raton: Chapman & Hall/CRC; 2004.
Tan MC, Linehan DC, Hawkins WG, Siegel BA, Strasberg SM. Chemotherapy-induced normalization of FDG uptake by colorectal liver metastases does not usually indicate complete pathologic response. J Gastrointest Surg 2007;11:1112–9.
Lubezky N, Metser U, Geva R, Nakache R, Shmueli E, Klausner JM, et al. The role and limitations of 18-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) scan and computerized tomography (CT) in restaging patients with hepatic colorectal metastases following neoadjuvant chemotherapy: comparison with operative and pathological findings. J Gastrointest Surg 2007;11:472–8.
Strauss LG, Dimitrakopoulou-Strauss A. Can PET-CT with FDG replace contrast enhanced CT for imaging of liver metastases? Eur J Nucl Med Mol Imaging 2007;34:1902–5.
Bipat S, van Leeuwen MS, Comans EF, Pijl ME, Bossuyt PM, Zwinderman AH, et al. Colorectal liver metastases: CT, MR imaging, and PET for diagnosis–meta-analysis. Radiology 2005;237:123–31.
Gronchi A, Fiore M, Miselli F, Lagonigro MS, Coco P, Messina A, et al. Surgery of residual disease following molecular-targeted therapy with imatinib mesylate in advanced/metastatic GIST. Ann Surg 2007;245:341–6.
Wardelmann E, Thomas N, Merkelbach-Bruse S, Pauls K, Speidel N, Büttner R, et al. Acquired resistance to imatinib in gastrointestinal stromal tumours caused by multiple KIT mutations. Lancet Oncol 2005;6:249–51.
Ye YJ, Gao ZD, Poston GJ, Wang S. Diagnosis and multi-disciplinary management of hepatic metastases from gastrointestinal stromal tumour (GIST). Eur J Surg Oncol 2009;35:787–92.
Prenen H, Stefan C, Landuyt B, Vermaelen P, Debiec-Rychter M, Bollen M, et al. Imatinib mesylate inhibits glucose uptake in gastrointestinal stromal tumor cells by downregulation of the glucose transporters recruitment to the plasma membrane. Am J Biochem Biotechnol 2005;1:95–102.
Klawitter J, Anderson N, Klawitter J, Christians U, Leibfritz D, Eckhardt SG, et al. Time-dependent effects of imatinib in human leukaemia cells: a kinetic NMR-profiling study. Br J Cancer 2009;100:923–31.
Shankar S, vanSonnenberg E, Desai J, DiPiro PJ, Van Den Abbeele A, Demetri GD. Gastrointestinal stromal tumor: new nodule-within-a-mass pattern of recurrence after partial response to imatinib mesylate. Radiology 2005;235:892–8.
Lammertsma AA, Hoekstra CJ, Giaccone G, Hoekstra OS. How should we analyse FDG PET studies for monitoring tumour response? Eur J Nucl Med Mol Imaging 2006;33 Suppl 1:16–21.
Torizuka T, Nobezawa S, Momiki S, Kasamatsu N, Kanno T, Yoshikawa E, et al. Short dynamic FDG-PET imaging protocol for patients with lung cancer. Eur J Nucl Med 2000;27:1538–42.
Strauss LG, Dimitrakopoulou-Strauss A, Haberkorn U. Shortened PET data acquisition protocol for the quantification of 18F-FDG kinetics. J Nucl Med 2003;44:1933–9.
Nitzsche EU, Hoegerle S, Mix M, Brink I, Otte A, Moser E, et al. Non-invasive differentiation of pancreatic lesions: is analysis of FDG kinetics superior to semiquantitative uptake value analysis? Eur J Nucl Med Mol Imaging 2002;29:237–42.
Dimitrakopoulou-Strauss A, Strauss LG, Heichel T, Wu H, Burger C, Bernd L, et al. The role of quantitative (18)F-FDG PET studies for the differentiation of malignant and benign bone lesions. J Nucl Med 2002;43:510–8.
Doot RK, Dunnwald LK, Schubert EK, Muzi M, Peterson LM, Kinahan PE, et al. Dynamic and static approaches to quantifying 18F-FDG uptake for measuring cancer response to therapy, including the effect of granulocyte CSF. J Nucl Med 2007;48:920–5.
Strauss LG, Koczan D, Klippel S, Pan L, Cheng C, Willis S, et al. Impact of angiogenesis-related gene expression on the tracer kinetics of 18F-FDG in colorectal tumors. J Nucl Med 2008;49:1238–44.
Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med 2009;50 Suppl 1:122S–50.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Apostolopoulos, D.J., Dimitrakopoulou-Strauss, A., Hohenberger, P. et al. Parametric images via dynamic 18F-fluorodeoxyglucose positron emission tomographic data acquisition in predicting midterm outcome of liver metastases secondary to gastrointestinal stromal tumours. Eur J Nucl Med Mol Imaging 38, 1212–1223 (2011). https://doi.org/10.1007/s00259-011-1776-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-011-1776-2