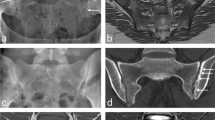
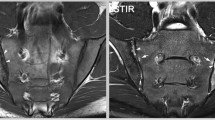
Abstract
Objective
The objective of this study was to evaluate the feasibility and reproducibility of high-resolution magnetic resonance imaging (MRI) and quantitative T2 mapping of the talocrural cartilage within a clinically applicable scan time using a new dedicated ankle coil and high-field MRI.
Materials and methods
Ten healthy volunteers (mean age 32.4 years) underwent MRI of the ankle. As morphological sequences, proton density fat-suppressed turbo spin echo (PD-FS-TSE), as a reference, was compared with 3D true fast imaging with steady-state precession (TrueFISP). Furthermore, biochemical quantitative T2 imaging was prepared using a multi-echo spin-echo T2 approach. Data analysis was performed three times each by three different observers on sagittal slices, planned on the isotropic 3D-TrueFISP; as a morphological parameter, cartilage thickness was assessed and for T2 relaxation times, region-of-interest (ROI) evaluation was done. Reproducibility was determined as a coefficient of variation (CV) for each volunteer; averaged as root mean square (RMSA) given as a percentage; statistical evaluation was done using analysis of variance.
Results
Cartilage thickness of the talocrural joint showed significantly higher values for the 3D-TrueFISP (ranging from 1.07 to 1.14 mm) compared with the PD-FS-TSE (ranging from 0.74 to 0.99 mm); however, both morphological sequences showed comparable good results with RMSA of 7.1 to 8.5%. Regarding quantitative T2 mapping, measurements showed T2 relaxation times of about 54 ms with an excellent reproducibility (RMSA) ranging from 3.2 to 4.7%.
Conclusion
In our study the assessment of cartilage thickness and T2 relaxation times could be performed with high reproducibility in a clinically realizable scan time, demonstrating new possibilities for further investigations into patient groups.




Similar content being viewed by others
References
Millington SA, Li B, Tang J, et al. Quantitative and topographical evaluation of ankle articular cartilage using high resolution MRI. J Orthop Res 2007; 25: 143–151.
Hangody L, Fules P. Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: ten years of experimental and clinical experience. J Bone Joint Surg Am 2003; 85–A(Suppl 2): 25–32.
Ba-Ssalamah A, Schibany N, Puig S, Herneth AM, Noebauer-Huhmann IM, Trattnig S. Imaging articular cartilage defects in the ankle joint with 3D fat-suppressed echo planar imaging: comparison with conventional 3D fat-suppressed gradient echo imaging. J Magn Reson Imaging 2002; 16: 209–216.
Al-Ali D, Graichen H, Faber S, Englmeier KH, Reiser M, Eckstein F. Quantitative cartilage imaging of the human hind foot: precision and inter-subject variability. J Orthop Res 2002; 20: 249–256.
Bolog N, Nanz D, Weishaupt D. Musculoskeletal MR imaging at 3.0 T: current status and future perspectives. Eur Radiol 2006; 16: 1298–1307.
Mosher TJ. Musculoskeletal imaging at 3T: current techniques and future applications. Magn Reson Imaging Clin N Am 2006; 14: 63–76.
Ramnath RR. 3T MR imaging of the musculoskeletal system. II. Clinical applications. Magn Reson Imaging Clin N Am 2006; 14: 41–62.
Schibany N, Ba-Ssalamah A, Marlovits S, et al. Impact of high field (3.0 T) magnetic resonance imaging on diagnosis of osteochondral defects in the ankle joint. Eur J Radiol 2005; 55: 283–288.
Antonio GE, Griffith JF, Yeung DK. Small-field-of-view MRI of the knee and ankle. AJR Am J Roentgenol 2004; 183: 24–28.
Eckstein F, Kunz M, Hudelmaier M, et al. Impact of coil design on the contrast-to-noise ratio, precision, and consistency of quantitative cartilage morphometry at 3 Tesla: a pilot study for the osteoarthritis initiative. Magn Reson Med 2007; 57: 448–454.
Barr C, Bauer JS, Malfair D, et al. MR imaging of the ankle at 3 Tesla and 1.5 Tesla: protocol optimization and application to cartilage, ligament and tendon pathology in cadaver specimens. Eur Radiol 2007; 17: 1518–1528.
Ramnath RR. 3T MR imaging of the musculoskeletal system. I. Considerations, coils, and challenges. Magn Reson Imaging Clin N Am 2006; 14: 27–40.
Burgkart R, Glaser C, Hyhlik-Durr A, Englmeier KH, Reiser M, Eckstein F. Magnetic resonance imaging-based assessment of cartilage loss in severe osteoarthritis: accuracy, precision, and diagnostic value. Arthritis Rheum 2001; 44: 2072–2077.
Glaser C, Faber S, Eckstein F, et al. Optimization and validation of a rapid high-resolution T1-w 3D FLASH water excitation MRI sequence for the quantitative assessment of articular cartilage volume and thickness. Magn Reson Imaging 2001; 19: 177–185.
Eckstein F, Hudelmaier M, Wirth W, et al. Double echo steady state magnetic resonance imaging of knee articular cartilage at 3 Tesla: a pilot study for the Osteoarthritis Initiative. Ann Rheum Dis 2006; 65: 433–441.
Friedrich KM, Reiter G, Kaiser B, et al. Cartilage imaging: fundamental evaluation of modern high-resolution isotropic 3D MR sequences at 3 T. Eur Radiol 2007; 17.
Weckbach S, Mendlik T, Horger W, Wagner S, Reiser MF, Glaser C. Quantitative assessment of patellar cartilage volume and thickness at 3.0 tesla comparing a 3D-fast low angle shot versus a 3D-true fast imaging with steady-state precession sequence for reproducibility. Invest Radiol 2006; 41: 189–197.
Burstein D, Velyvis J, Scott KT, et al. Protocol issues for delayed Gd(DTPA)(2-)-enhanced MRI: (dGEMRIC) for clinical evaluation of articular cartilage. Magn Reson Med 2001; 45: 36–41.
Miller KL, Hargreaves BA, Gold GE, Pauly JM. Steady-state diffusion-weighted imaging of in vivo knee cartilage. Magn Reson Med 2004; 51: 394–398.
Trattnig S, Millington SA, Szomolanyi P, Marlovits S. MR imaging of osteochondral grafts and autologous chondrocyte implantation. Eur Radiol 2007; 17: 103–118.
Watrin-Pinzano A, Ruaud JP, Cheli Y, et al. Evaluation of cartilage repair tissue after biomaterial implantation in rat patella by using T2 mapping. Magma 2004; 17: 219–228.
White LM, Sussman MS, Hurtig M, Probyn L, Tomlinson G, Kandel R. Cartilage T2 assessment: differentiation of normal hyaline cartilage and reparative tissue after arthroscopic cartilage repair in equine subjects. Radiology 2006; 241: 407–414.
Williams A, Gillis A, McKenzie C, et al. Glycosaminoglycan distribution in cartilage as determined by delayed gadolinium-enhanced MRI of cartilage (dGEMRIC): potential clinical applications. Am J Roentgenol 2004; 182: 167–172.
Glaser C. New techniques for cartilage imaging: T2 relaxation time and diffusion-weighted MR imaging. Radiol Clin North Am 2005; 43: 641–653.
Steinhagen J, Niggemeyer O, Bruns J. [Etiology and pathogenesis of osteochondrosis dissecans tali]. Orthopade 2001; 30: 20–27.
Dietrich O, Raya JG, Reeder SB, Reiser MF, Schoenberg SO. Measurement of signal-to-noise ratios in MR images: influence of multichannel coils, parallel imaging, and reconstruction filters. J Magn Reson Imaging 2007; 26: 375–385.
Firbank MJ, Coulthard A, Harrison RM, Williams ED. A comparison of two methods for measuring the signal to noise ratio on MR images. Phys Med Biol 1999; 44: N261–N264.
Li T, Mirowitz SA. Fast multi-planar gradient echo MR imaging: impact of variation in pulse sequence parameters on image quality and artifacts. Magn Reson Imaging 2004; 22: 807–814.
Noebauer-Huhmann IM, Glaser C, Dietrich O, et al. MR imaging of the cervical spine: assessment of image quality with parallel imaging compared to non-accelerated MR measurements. Eur Radiol 2007; 17: 1147–1155.
Gluer CC, Blake G, Lu Y, Blunt BA, Jergas M, Genant HK. Accurate assessment of precision errors: how to measure the reproducibility of bone densitometry techniques. Osteoporos Int 1995; 5: 262–270.
Blaimer M, Breuer F, Mueller M, Heidemann RM, Griswold MA, Jakob PM. SMASH, SENSE, PILS, GRAPPA: how to choose the optimal method. Top Magn Reson Imaging 2004; 15: 223–236.
Bauer JS, Banerjee S, Henning TD, Krug R, Majumdar S, Link TM. Fast high-spatial-resolution MRI of the ankle with parallel imaging using GRAPPA at 3 T. AJR Am J Roentgenol 2007; 189: 240–245.
Tan TC, Wilcox DM, Frank L, et al. MR imaging of articular cartilage in the ankle: comparison of available imaging sequences and methods of measurement in cadavers. Skeletal Radiol 1996; 25: 749–755.
Trattnig S, Breitenseher MJ, Huber M, et al. [Determination of cartilage thickness in the ankle joint. an MRT (1.5)-anatomical comparative study]. Rofo 1997; 166: 303–306.
Eckstein F, Reiser M, Englmeier KH, Putz R. In vivo morphometry and functional analysis of human articular cartilage with quantitative magnetic resonance imaging-from image to data, from data to theory. Anat Embryol (Berl) 2001; 203: 147–173.
Mintz DN, Tashjian GS, Connell DA, Deland JT, O’Malley M, Potter HG. Osteochondral lesions of the talus: a new magnetic resonance grading system with arthroscopic correlation. Arthroscopy 2003; 19: 353–359.
Dardzinski BJ, Mosher TJ, Li S, Van Slyke MA, Smith MB. Spatial variation of T2 in human articular cartilage. Radiology 1997; 205: 546–550.
Kurkijarvi JE, Nissi MJ, Ojala RO, et al. In vivo T2 mapping and dGEMRIC of human articular cartilage repair after autologous chondrocyte transplantation. Proc Intl Soc Magn Reson Med 2005; 13: 481.
Mosher TJ, Dardzinski BJ, Smith MB. Human articular cartilage: influence of aging and early symptomatic degeneration on the spatial variation of T2—preliminary findings at 3 T. Radiology 2000; 214: 259–266.
Mosher TJ, Smith H, Dardzinski BJ, Schmithorst VJ, Smith MB. MR imaging and T2 mapping of femoral cartilage: in vivo determination of the magic angle effect. AJR Am J Roentgenol 2001; 177: 665–669.
Mosher TJ, Smith HE, Collins C, et al. Change in knee cartilage T2 at MR imaging after running: a feasibility study. Radiology 2005; 234: 245–249.
Dunn TC, Lu Y, Jin H, Ries MD, Majumdar S. T2 relaxation time of cartilage at MR imaging: comparison with severity of knee osteoarthritis. Radiology 2004; 232: 592–598.
Koff MF, Amrami KK, Kaufman KR. Clinical evaluation of T2 values of patellar cartilage in patients with osteoarthritis. Osteoarthritis Cartilage 2007; 15: 198–204.
Link TM, Stahl R, Woertler K. Cartilage imaging: motivation, techniques, current and future significance. Eur Radiol 2007; 17: 1135–1146.
Mosher TJ, Dardzinski BJ. Cartilage MRI T2 relaxation time mapping: overview and applications. Semin Musculoskelet Radiol 2004; 8: 355–368.
Welsch GH, Mamisch TC, Salomonowitz E, et al. Quantitative T2 mapping of matrix-associated autologous chondrocyte transplantation: an in vivo follow-up study. Eur Radiol 2007; 17: 291.
Dorotka R, Kotz R, Trattnig S, Nehrer S. [Mid-term results of autologous chondrocyte transplantation in knee and ankle. A one- to six-year follow-up study]. Z Rheumatol 2004; 63: 385–392.
Giannini S, Buda R, Grigolo B, Vannini F, De Franceschi L, Facchini A. The detached osteochondral fragment as a source of cells for autologous chondrocyte implantation (ACI) in the ankle joint. Osteoarthritis Cartilage 2005; 13: 601–607.
Gigante A, Bevilacqua C, Ricevuto A, Mattioli-Belmonte M, Greco F. Membrane-seeded autologous chondrocytes: cell viability and characterization at surgery. Knee Surg Sports Traumatol Arthrosc 2007; 15: 88–92.
Koulalis D, Schultz W, Heyden M. Autologous chondrocyte transplantation for osteochondritis dissecans of the talus. Clin Orthop Relat Res 2002; 395: 186–192.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Welsch, G.H., Mamisch, T.C., Weber, M. et al. High-resolution morphological and biochemical imaging of articular cartilage of the ankle joint at 3.0 T using a new dedicated phased array coil: in vivo reproducibility study. Skeletal Radiol 37, 519–526 (2008). https://doi.org/10.1007/s00256-008-0474-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-008-0474-z