Abstract
Background
T1 relaxation time is a potential magnetic resonance imaging (MRI) biomarker for fibrosis and inflammation of the solid abdominal organs. However, normal T1 relaxation times of the solid abdominal organs have not been defined for children.
Objective
The purpose of this study was to measure T1 relaxation times of the liver, pancreas and spleen in healthy children.
Materials and methods
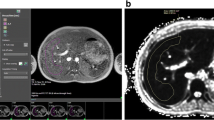
This was an institutional review board-approved study of a convenience sample of prospectively recruited, healthy children ages 7 to 17 years undergoing research abdominal MRI (1.5 or 3 T) as part of a larger research study between February 2018 and October 2018. For the current study, T1 mapping was performed with a Modified Look-Locker sequence covering the upper abdomen. A single reviewer placed freehand regions of interest on the T1 parametric maps in the liver, pancreas and spleen, inclusive of as much parenchyma as possible. Student’s t-tests and linear regression were used to compare T1 values by age and gender.
Results
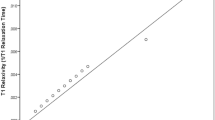
Thirty-two participants were included (16 female:16 male; mean age: 12.2±3.1 years; n=16 at 1.5 T). Median T1 relaxation times (ms) per organ were liver: 581±64 (1.5 T), 783±88 (3 T); pancreas: 576±55 (1.5 T), 730±30 (3 T), and spleen: 1,172±71 (1.5 T), 1,356±87 (3 T). T1 values were not statistically significantly different between males and females. At both 1.5 and 3 T field strengths, linear regression showed no significant association between age and T1 values for the liver, pancreas and spleen.
Conclusion
We report normal T1 relaxation times for the liver, pancreas and spleen at 1.5 and 3 T in a cohort of healthy children.

Similar content being viewed by others
References
Adams LA, Lymp JF, St Sauver J et al (2005) The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology 129:113–121
Serai SD, Trout AT, Miethke A et al (2018) Putting it all together: established and emerging MRI techniques for detecting and measuring liver fibrosis. Pediatr Radiol 48:1256–1272
Taylor AJ, Salerno M, Dharmakumar R, Jerosch-Herold M (2016) T1 mapping: basic techniques and clinical applications. JACC Cardiovasc Imaging 9:67–81
Luetkens JA, Klein S, Traber F et al (2018) Quantification of liver fibrosis at T1 and T2 mapping with extracellular volume fraction MRI: preclinical results. Radiology 288:748–754
Banerjee R, Pavlides M, Tunnicliffe EM et al (2014) Multiparametric magnetic resonance for the non-invasive diagnosis of liver disease. J Hepatol 60:69–77
Haimerl M, Verloh N, Fellner C et al (2014) MRI-based estimation of liver function: Gd-EOB-DTPA-enhanced T1 relaxometry of 3T vs. the MELD score. Sci Rep 4:5621
Pavlides M, Banerjee R, Sellwood J et al (2016) Multiparametric magnetic resonance imaging predicts clinical outcomes in patients with chronic liver disease. J Hepatol 64:308–315
Pavlides M, Banerjee R, Tunnicliffe EM et al (2017) Multiparametric magnetic resonance imaging for the assessment of non-alcoholic fatty liver disease severity. Liver Int 37:1065–1073
Tunnicliffe EM, Banerjee R, Pavlides M et al (2017) A model for hepatic fibrosis: the competing effects of cell loss and iron on shortened modified look-locker inversion recovery T1 (shMOLLI-T1 ) in the liver. J Magn Reson Imaging 45:450–462
Zhou ZP, Long LL, Qiu WJ et al (2017) Evaluating segmental liver function using T1 mapping on Gd-EOB-DTPA-enhanced MRI with a 3.0 tesla. BMC Med Imaging 17:20
Mojtahed A, Kelly CJ, Herlihy AH et al (2019) Reference range of liver corrected T1 values in a population at low risk for fatty liver disease-a UK biobank sub-study, with an appendix of interesting cases. Abdom Radiol (NY) 44:72–84
Tirkes T, Lin C, Fogel EL et al (2017) T1 mapping for diagnosis of mild chronic pancreatitis. J Magn Reson Imaging 45:1171–1176
Tirkes T, Lin C, Cui E et al (2018) Quantitative MR evaluation of chronic pancreatitis: extracellular volume fraction and MR Relaxometry. AJR Am J Roentgenol 210:533–542
Wang M, Gao F, Wang X et al (2018) Magnetic resonance elastography and T1 mapping for early diagnosis and classification of chronic pancreatitis. J Magn Reson Imaging. https://doi.org/10.1002/jmri.26008
Liu A, Wijesurendra RS, Ariga R et al (2017) Splenic T1-mapping: a novel quantitative method for assessing adenosine stress adequacy for cardiovascular magnetic resonance. J Cardiovasc Magn Reson 19(1)
Lee DH, Lee JM, Han JK, Choi BI (2013) MR elastography of healthy liver parenchyma: Normal value and reliability of the liver stiffness value measurement. J Magn Reson Imaging 38:1215–1223
Bull S, White SK, Piechnik SK et al (2013) Human non-contrast T1 values and correlation with histology in diffuse fibrosis. Heart 99:932–937
Flett AS, Hayward MP, Ashworth MT et al (2010) Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: preliminary validation in humans. Circulation 122:138–144
Iles L, Pfluger H, Phrommintikul A et al (2008) Evaluation of diffuse myocardial fibrosis in heart failure with cardiac magnetic resonance contrast-enhanced T1 mapping. J Am Coll Cardiol 52:1574–1580
Miller CA, Naish JH, Bishop P et al (2013) Comprehensive validation of cardiovascular magnetic resonance techniques for the assessment of myocardial extracellular volume. Circ Cardiovasc Imaging 6:373–383
Ferreira VM, Piechnik SK, Dall'Armellina E et al (2014) Native T1-mapping detects the location, extent and patterns of acute myocarditis without the need for gadolinium contrast agents. J Cardiovasc Magn Reson 16:36
Messroghli DR, Greiser A, Frohlich M et al (2007) Optimization and validation of a fully-integrated pulse sequence for modified look-locker inversion-recovery (MOLLI) T1 mapping of the heart. J Magn Reson Imaging 26:1081–1086
Messroghli DR, Radjenovic A, Kozerke S et al (2004) Modified look-locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn Reson Med 52:141–146
Piechnik SK, Ferreira VM, Dall'Armellina E et al (2010) Shortened modified look-locker inversion recovery (ShMOLLI) for clinical myocardial T1-mapping at 1.5 and 3 T within a 9 heartbeat breathhold. J Cardiovasc Magn Reson 12:69
Carrick D, Haig C, Rauhalammi S et al (2016) Prognostic significance of infarct core pathology revealed by quantitative non-contrast in comparison with contrast cardiac magnetic resonance imaging in reperfused ST-elevation myocardial infarction survivors. Eur Heart J 37:1044–1059
Chan W, Duffy SJ, White DA et al (2012) Acute left ventricular remodeling following myocardial infarction: coupling of regional healing with remote extracellular matrix expansion. JACC Cardiovasc Imaging 5:884–893
Puntmann VO, Voigt T, Chen Z et al (2013) Native T1 mapping in differentiation of normal myocardium from diffuse disease in hypertrophic and dilated cardiomyopathy. JACC Cardiovasc Imaging 6:475–484
Hinojar R, Foote L, Arroyo Ucar E et al (2015) Native T1 in discrimination of acute and convalescent stages in patients with clinical diagnosis of myocarditis: a proposed diagnostic algorithm using CMR. JACC Cardiovasc Imaging 8:37–46
Bjornerud A, Johansson LO, Briley-Saebo K, Ahlstrom HK (2002) Assessment of T1 and T2* effects in vivo and ex vivo using iron oxide nanoparticles in steady state--dependence on blood volume and water exchange. Magn Reson Med 47:461–471
Acknowledgments
This research was supported by the Society for Pediatric Radiology (SPR) Research & Education Foundation. An institutional review board approved this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Andrew Trout has unrelated research grants from Canon Medical Systems, receives in-kind support for research from Perspectum Diagnostics and Siemens Medical Solutions, is part of the Speakers Bureau in applied radiology, and receives honoraria for authorship from Reed Elsevier and Wolters Kluwer.
Dr. Jonathan Dillman shares Canon, Siemens and Perspectum COIs w/ Dr. Trout
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gilligan, L.A., Dillman, J.R., Tkach, J.A. et al. Magnetic resonance imaging T1 relaxation times for the liver, pancreas and spleen in healthy children at 1.5 and 3 tesla. Pediatr Radiol 49, 1018–1024 (2019). https://doi.org/10.1007/s00247-019-04411-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-019-04411-7