Abstract
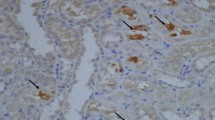
Renal tubular epithelium is the major target for oxalate induced injury, and sustained hyperoxaluria together with CaOx crystal formation/deposition may induce renal tubular cell damage and/or dysfunction. This may express itself in cell apoptosis. To evaluate the possible protective effects of certain agents (vitamin E, potassium citrate, allopurinol, verapamil and MgOH) on the presence and the severity of apoptotic changes caused by hyperoxaluria on renal tubular epithelium, an experimental study in rabbits was performed. Seventy rabbits were divided into seven different groups (each group n=10): in group I severe hyperoxaluria was induced by continuous ethylene glycol (0.75%) administration started on day 0 and completed on day 14. Histologic alterations including crystal formation together with apoptotic changes (by using the TUNEL method) were evaluated on days 21 and 42, respectively. In the remaining experimental groups (groups II–VI), animals received some agents in addition to the induction of hyperoxaluria in an attempt to limit apoptotic changes. Group VII) animals constituted the controls. Kidneys were examined histopathologically under light microscopy for the presence and degree of crystal deposition in the tubular lumen. The percentage of apoptotic nuclei in the control group was significantly different from the other group animals (2.9–2.4%) in all study phases (P<0.05). Apart from potassium citrate and allopurinol, the other medications seemed to prevent or limit the formation of apoptotic changes in renal tubular epithelium during the early period (day 21). The percentage of positively stained nuclei in animals undergoing potassium citrate medication ranged from 24.3% to 28.6%, with an average of 27.1%. This was 18.4% in animals receiving allopurinol. On the other hand, animals receiving magnesium hydroxide (MgOH), verapamil and vitamin E demonstrated limited apoptotic changes (11.2, 9.7, 8.7%, respectively) during this phase(P<0.05). In the long-term (day 42), the animals receiving allopurinol and eitamine E showed a decrease in the percentage of the positively stained nuclei (13.5% and 8.3%, respectively). Animals in the other groups showed an increase in the number and percentage of apoptotic cells. Although, there was a significant decrease in the mean values of apoptosis in animals receiving vitamine E (8.7%–8.3%) and allopurinol (18.4%–13.5%) (P<0.05), animals on verapamil, MgOH and potassium citrate medication had an increase in these values or the change was not found to be significant. In the light of our findings and results from the literature, it is clear that that both hyperoxaluria and CaOx crystals may be injurious to renal epithelial cells. Apoptotic changes observed in renal tubular epithelial cells induced by massive hyperoxaluria might result in cell degradation and may play a role in the pathologic course of urolithiasis. Again, as demonstrated in our study, the limitation of both crystal deposition and apoptotic changes might be instituted by some antioxidant agents as well as urinary inhibitors. Clinical application of such agents in the prophylaxis of stone disease might limit the formation of urinary calculi, especially in recurrent stone formers.



Similar content being viewed by others
References
Agarwal A, İkemoto I, Loughlin KR (1997) Prevention of testicular damage by free radical scavengers. Urology 50: 759
Akbay C, Sayın C, Sarıca K, Soygür T, Sabuncuoğlu B (1998) Effect of verapamil on rabbit renal tissue after shock wave lithotripsy: an ultrastructural approach. Electron Microsc 4: 493
Anuradha CV, Selvam R (1989) Increased lipid peroxidation in the erythrocytes of kidney stone formers. Indian J Biochem Biophys 26: 39
Benyi L, Weilherg Z, Puyun L (1995) Protective effects of nifedipine and allopurinol on high energy shock wave induced acute changes of renal function. J Urol 153: 596
Biri H, Öztürk HS, Büyükkoçak S, Kaçmaz M (1998) Antioxidant defense potential of rabbit renal tissues after ESWL: protective effect of antioxidant vitamins Nephron 79: 181
Cohen PJ (1992) Allopurinol administered prior to hepatic ischemia in the rat prevents chemiluminescence folloin restoration of circulation. Can J Anesth 39: 1090
De Water R, Boeve ER, Van Miert PP, Vermaire CP, Van Run PR, Cao LC, De Bruijn WC, Schroder FH (1996) Pathological and immunocytochemical changes in chronic calcium oxalate neprolithiasis in the rat. Scanning Microsc 10: 577
Finlayson B (1978) Physicochemical aspects of urolithiasis. Kidney Int 13: 344
Hackett RL, Shevock PN, Khan SR (1990) Cell injury associated with calcium oxalate crystalluria. J Urol 144: 1535
Hackett RL, Shevock PN, Khan SR (1994) Madin-Darby canine kidney cells are injured by exposure to oxalate and to calcium oxalate crystals. Urol Res 22: 197
Hackett RL, Shevock PN, Khan SR (1995) Alterations in MDCK and LLC-PK1 cells exposed to oxalate and calcium oxalate monohydrate crystals. Scanning Micorosc 9: 587
Khan SR (1995) Calcium oxalate crystal interaction with renal tubular epithelium, mechanism of crystal adhesion and its impact on stone development. Urol Res 23: 71
Khan SR, Hackett RL (1993) Hyperoxaluria, enzymuria and nephrolithiasis. Contrib Nephrol 101: 190
Khan SR, Shevock PN, Hackett RL (1989) Urinary enzymes and calcium oxalate urolithiasis. J Urol 142: 846
Khan SR, Shevock PN, Hackett RL (1992) Acute hyperoxaluria, renal injury and calcium oxalete urolithiasis. J Urol 147: 226
Khan SR, Shevock PN, Hackett RL (1993) Magnesium oxide administration and prevention of calcium oxalate nephrolithiasis. J Urol 149: 412
Kohri K, Garside J, Blacklock NJ (1988)The role of magnesium in calcium oxalate urolithiasis. Br J Urol 61: 107
Koul H, Kennington L, Honeyman T, Jonassen J, Menon M, Scheid C(1996) Activation of c-myc gene mediates the mitogenic effects of oxalate in LLC-PK1 cells, a line of renal epithelil cells. Kidney Int 50: 1525
Koul H, Kennington L, Nair G, Honeyman T, Menon M, Scheid C (1994) Oxalate-induced initiation of DNA synthesis in LLC-PK1 cells, a line of renal epithelial cells. Biochem Biophys Res Commun 205: 1632
Koul S, Fu S, Menon M, Koul H(2000) Oxalate exposure induces apoptosis in renal proximal tubular epithelial cells (LLC-PK1 and HK-2 cells) in culture. Urolithiasis 2000, 9th International Symposium on Urolithiasis, Proceedings, p 247
Lieske JC, Norris R, Swift H, Toback FG(1997) Adhesion, internalization and metabolism of calcium oxalate monohydrate crystals by renal epithelial cells. Kidney Int 52: 1291
Mandel N (1994) Crystal-membrane interaction in kidney stone disease. J Am Soc Nephrol 5: 37
Miyazawa K, Suzuki K, Ueda Y, Katsuda S (2000) Demonstration of apoptosis and its related genes in rat tubular epithelium of calcium oxalate crystal formation. Urolithiasis 2000, 9th International Symposium on Urolithiasis, Proceedings, p 253
Ogawa Y, Yamaguchi K, Morozumi M (1990) Effects of magnesium salts in preventing experimental oxalate urolithiasis. J Urol, 144: 385
Parekh MH, Lobel R, O’Connor L, Leggett RE, Levin RM (2001) Protective effect of Vitamin E on the response of the rabbit bladder to partial outlet obstruction. J Urol 166: 341
Reilly P, Schiller HJ, Bulkley GB (1992) Pharmacologic approach to tissue injury mediated by free radicals and other reactive oxygen metabolites. Am J Surg 161: 488
Sarıca K, Bakır K, Yağcı F, Erbağcı A, Topçu O, Uysal O (2000) Unilateral testicular torsion; protective effect of verapamil on contralateral testicular histology. Urol Int 62: 159
Sarıca K, Bakır K, Yağcı F, Topçu O, Akbay C, Sayın N, Korkmaz C (1999) Limitation of possible enhanced crystal deposition by verapamil in renal parenchyma after shock wave application in rabbit model. J Endourol 13: 343
Sarıca K, Koşar A, Yaman Ö, Bedük Y, Durak İ, Göğüş O, Kavukçu M (1996) Evaluation of ischemia after ESWL: detection of free oxygen radical scavenger enzymes in renal parenchyma subjected to high energy shock waves. Urol Int 57: 221
Sarıca K, Küpeli B, Budak M, Koşar A, Kavukçu M, Durak İ, Göğüş O (1997) Influence of experimental spermatic cord torsion on the contralateral testes in rats: evaluation of tissue free oxygen scavenger enzyme levels. Urol Int 58: 208
Sarıca K, Özer G, Soygür T, Yaman Ö, Özer E, Üstün H, Yaman LS, Göğüş O (1997) Preservation of shock-wave-induced renal histologic changes by Dermatan sulphate. Urology 49: 145
Sarıca K, Yağcı F, Bakır K, Erturhan S, Uçak R (2001) Renal tubular injury induced by hyperoxaluria: evaluation of apoptotic changes. Urol Res 29: 34
Scheid C, Koul H, Hill WA, Luber-Narod J, Kennington L, Honeyman T, Jonassen J, Menon M (1996) Oxalate toxicity in LLC-PK1 cells:role of free radicals. Kidney Int 49: 413
Scheid CR, Koul H, Hill WA, Luber-Narod J, Jonassen J, Honeyman T, Kennington L, Kohli R, Hodapp J, Ayvazian P, Menon M (1996) Oxalate toxicity in in LLC-PK1 cells, a line of renal epithelial cells. J Urol 155: 1112
Scheid CR, Koul H, Kennington L, Hill WA, Luber-Narod J, Jonassen J, Honeyman T, Menon M (1995) Oxalate-induced damage to renal tubular cells. Scanning Microsc 9: 1097
Selvam R, Adhirai M (1997) Vitamin E pretreatment prevents Cyclosporin A induced crystal deposition in hyperoxaluric rats. Nephron 75: 77
Selvam R, Bijikurien T (1992) Effect of citrate feeding on free radical induced changes in experimental urolithiasis. Indian J Exp Biol 30: 705
Strohmaier WL, Abelius A, Billes J, Grossmann T, Wilbert DM, Bichler KH (1994) Verapamil limits shock wave-induced renal tubular damage in vivo. J Endourol 8: 269
Strohmaier WL, Bichler KH, Koch J, Balk KN, Wilbert DM (1993) Protective effect of verapamil on shock wave-induced renal tubular dysfunction. J Urol 150: 27
Su CJ, Shevock PN, Khan SR, Hackett RL (1991) Effect of magnesium on calcium oxalate urolithiasis. J Urol, 45: 1092
Thamilselvan S, Hackett RL, Khan SR (1997)Lipid peroxidation in ethylene glycol induced hyperoxaluria and calcium oxalate nephrolithiasis. J Urol 157: 1059
Thamilselvan S, Khan SR (1998) Oxalate and calcium oxalate crystals are injurious to renal epithelial cells: results of in vivo and in vitro studies. J Nephrol 11: 66
Vaughan WG, Horton JW, Walker PB (1992) Allopurinol prevents intestinal permeability changes after ischemia-reperfusion injury. J Pediatr Surg 27: 968
Wu SH, Oldfield JE, Whanger PD, Weswig PH (1973) Effect of selenium, vitamin E and antioxidants on testicular function in rats. Biol Reprod 8: 625
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sarica, K., Erbagci, A., Yağci, F. et al. Limitation of apoptotic changes in renal tubular cell injury induced by hyperoxaluria. Urol Res 32, 271–277 (2004). https://doi.org/10.1007/s00240-003-0393-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-003-0393-3