Abstract
Background
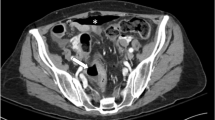
The aim of this meta-analysis was to gather current data and evaluate not only the risk of gastrointestinal (GI) perforation with bevacizumab, but also the potential risk factors for this adverse event.
Materials and methods
We carried out a literature search in PubMed for randomized controlled trials (RCTs) reported from January 2000 to December 2013. Summary incidence, relative risks (RRs) and 95 % confidence intervals (CIs) were calculated using random-effects or fixed-effects models based on the heterogeneity of the included studies.
Results
A total of 26,833 patients from 33 RCTs were included in the meta-analysis. Bevacizumab-containing therapy significantly increased the risk of developing all-grade (RR 3.35, 95 % CI 2.35–4.79, P < 0.001) and fatal GI perforation (RR 3.08, 95%CI: 1.04–9.08, P = 0.042). On subgroup analysis, no significant risk differences were found based on bevacizumab dosage, treatment duration, treatment line, type of clinical trial and median age. When stratified by tumor types, a significantly increased risk of GI perforation with bevacizumab was observed in colorectal cancer (RR 2.84, 95% CI 1.43–5.61, P = 0.003), gynecologic cancer (RR 3.37, 95% CI 1.71–6.62, P < 0.001) and prostate cancer (RR 6.01, 95% CI 1.78–20.28, P = 0.004). Additionally, the use of bevacizumab significantly increased the risk of GI perforation when used in conjunction with taxanes (RR 3.09, 95% CI 1.92–4.96, P < 0.001) or oxaliplatin (RR 2.85, 95% CI 1.07–7.57, P = 0.036).
Conclusions
Bevacizumab treatment is associated with a significantly increased risk of developing GI perforation, and clinicians should be aware of the risks of GI perforation with the administration of this drug in cancer patients.




Similar content being viewed by others
References
Folkman J (1971) Tumor angiogenesis: therapeutic implications. N Engl J Med 285(21):1182–1186. doi:10.1056/NEJM197111182852108
Folkman J (2002) Role of angiogenesis in tumor growth and metastasis. Semin Oncol 29[6 Suppl 16]:15–18. doi:10.1053/sonc.2002.37263
Hicklin DJ, Ellis LM (2005) Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol 23(5):1011–1027. doi:10.1200/JCO.2005.06.081
Cohen MH, Gootenberg J, Keegan P, Pazdur R (2007) FDA drug approval summary: bevacizumab plus FOLFOX4 as second-line treatment of colorectal cancer. Oncologist 12(3):356–361. doi:10.1634/theoncologist.12-3-356
Kabbinavar FF, Schulz J, McCleod M, Patel T, Hamm JT, Hecht JR, Mass R, Perrou B, Nelson B, Novotny WF (2005) Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: results of a randomized phase II trial. J Clin Oncol 23(16):3697–3705. doi:10.1200/JCO.2005.05.112
Cohen MH, Gootenberg J, Keegan P, Pazdur R (2007) FDA drug approval summary: bevacizumab (Avastin) plus Carboplatin and Paclitaxel as first-line treatment of advanced/metastatic recurrent nonsquamous non-small cell lung cancer. Oncologist 12(6):713–718. doi:10.1634/theoncologist.12-6-713
Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, Mannel RS, Homesley HD, Fowler J, Greer BE, Boente M, Birrer MJ, Liang SX (2011) Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med 365(26):2473–2483. doi:10.1056/NEJMoa1104390
Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, Yung WK, Paleologos N, Nicholas MK, Jensen R, Vredenburgh J, Huang J, Zheng M, Cloughesy T (2009) Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 27(28):4733–4740. doi:10.1200/JCO.2008.19.8721
Rini BI, Halabi S, Rosenberg JE, Stadler WM, Vaena DA, Ou SS, Archer L, Atkins JN, Picus J, Czaykowski P, Dutcher J, Small EJ (2008) Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206. J Clin Oncol 26(33):5422–5428. doi:10.1200/jco.2008.16.9847
Geiger-Gritsch S, Stollenwerk B, Miksad R, Guba B, Wild C, Siebert U (2010) Safety of bevacizumab in patients with advanced cancer: a meta-analysis of randomized controlled trials. Oncologist 15(11):1179–1191. doi:10.1634/theoncologist.2009-0155
Zhu X, Wu S, Dahut WL, Parikh CR (2007) Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: systematic review and meta-analysis. Am J Kidney Dis 49(2):186–193. doi:10.1053/j.ajkd.2006.11.039
Hapani S, Chu D, Wu S (2009) Risk of gastrointestinal perforation in patients with cancer treated with bevacizumab: a meta-analysis. Lancet Oncol 10(6):559–568. doi:10.1016/S1470-2045(09)70112-3
Vervenne W, Bennouna J, Humblet Y, Gill S, Moore MJ, Van Laethem J, Shang A, Cosaert J, Verslype C, Van Cutsem E (2008) A randomized, double-blind, placebo (P) controlled, multicenter phase III trial to evaluate the efficacy and safety of adding bevacizumab (B) to erlotinib (E) and gemcitabine (G) in patients (pts) with metastatic pancreatic cancer. J Clin Oncol 26(15s)[Suppl}: abstr 4057
Van Cutsem E, Vervenne WL, Bennouna J, Humblet Y, Gill S, Van Laethem JL, Verslype C, Scheithauer W, Shang A, Cosaert J, Moore MJ (2009) Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J Clin Oncol 27(13):2231–2237. doi:10.1200/jco.2008.20.0238
Aghajanian C, Blank SV, Goff BA, Judson PL, Teneriello MG, Husain A, Sovak MA, Yi J, Nycum LR (2012) OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol 30(17):2039–2045. doi:10.1200/JCO.2012.42.0505
Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G, Carey MS, Beale P, Cervantes A, Kurzeder C, du Bois A, Sehouli J, Kimmig R, Stahle A, Collinson F, Essapen S, Gourley C, Lortholary A, Selle F, Mirza MR, Leminen A, Plante M, Stark D, Qian W, Parmar MK, Oza AM (2011) A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med 365(26):2484–2496. doi:10.1056/NEJMoa1103799
Kelly WK, Halabi S, Carducci M, George D, Mahoney JF, Stadler WM, Morris M, Kantoff P, Monk JP, Kaplan E, Vogelzang NJ, Small EJ (2012) Randomized, double-blind, placebo-controlled phase III trial comparing docetaxel and prednisone with or without bevacizumab in men with metastatic castration-resistant prostate cancer: CALGB 90401. J Clin Oncol 30(13):1534–1540. doi:10.1200/jco.2011.39.4767
Ohtsu A, Shah MA, Van Cutsem E, Rha SY, Sawaki A, Park SR, Lim HY, Yamada Y, Wu J, Langer B, Starnawski M, Kang YK (2011) Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol 29(30):3968–3976. doi:10.1200/jco.2011.36.2236
National Cancer Institute (NCI) Cancer Therapy Evaluation Program (CTEP). CTC v 2.0 and common terminology criteria for adverse events criteria V3.0 (CTCAE). Available at: http://ctepcancergov/protocolDevelopment/electronic_applications/ctchtm. Accessed 27Jan 27 2013
Choueiri TK, Schutz FA, Je Y, Rosenberg JE, Bellmunt J (2010) Risk of arterial thromboembolic events with sunitinib and sorafenib: a systematic review and meta-analysis of clinical trials. J Clin Oncol 28(13):2280–2285. doi:10.1200/JCO.2009.27.2757
Zintzaras E, Ioannidis JP (2005) Heterogeneity testing in meta-analysis of genome searches. Genet Epidemiol 28(2):123–137. doi:10.1002/gepi.20048
Sterne JA, Gavaghan D, Egger M (2000) Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol 53(11):1119–1129
Bennouna J, Sastre J, Arnold D, Osterlund P, Greil R, Van Cutsem E, von Moos R, Vieitez JM, Bouche O, Borg C, Steffens CC, Alonso-Orduna V, Schlichting C, Reyes-Rivera I, Bendahmane B, Andre T, Kubicka S, Investigators MLS (2013) Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol 14(1):29–37. doi:10.1016/S1470-2045(12)70477-1
de Gramont A, Van Cutsem E, Schmoll H-J, Tabernero J, Clarke S, Moore MJ, Cunningham D, Cartwright TH, Hecht JR, Rivera F, Im S-A, Bodoky G, Salazar R, Maindrault-Goebel F, Shacham-Shmueli E, Bajetta E, Makrutzki M, Shang A, André T, Hoff PM (2012) Bevacizumab plus oxaliplatin-based chemotherapy as adjuvant treatment for colon cancer (AVANT): a phase 3 randomised controlled trial. Lancet Oncol 13(12):1225–1233. doi:10.1016/s1470-2045(12)70509-0
Guan ZZ, Xu JM, Luo RC, Feng FY, Wang LW, Shen L, Yu SY, Ba Y, Liang J, Wang D, Qin SK, Wang JJ, He J, Qi C, Xu RH (2011) Efficacy and safety of bevacizumab plus chemotherapy in Chinese patients with metastatic colorectal cancer: a randomized phase III ARTIST trial. Chin J Cancer 30(10):682–689. doi:10.5732/cjc.011.10188
Tebbutt NC, Wilson K, Gebski VJ, Cummins MM, Zannino D, van Hazel GA, Robinson B, Broad A, Ganju V, Ackland SP, Forgeson G, Cunningham D, Saunders MP, Stockler MR, Chua Y, Zalcberg JR, Simes RJ, Price TJ (2010) Capecitabine, bevacizumab, and mitomycin in first-line treatment of metastatic colorectal cancer: results of the Australasian Gastrointestinal Trials Group Randomized Phase III MAX Study. J Clin Oncol 28(19):3191–3198. doi:10.1200/jco.2009.27.7723
Allegra CJ, Yothers G, O’Connell MJ, Sharif S, Colangelo LH, Lopa SH, Petrelli NJ, Goldberg RM, Atkins JN, Seay TE, Fehrenbacher L, O’Reilly S, Chu L, Azar CA, Wolmark N (2009) Initial safety report of NSABP C-08: a randomized phase III study of modified FOLFOX6 with or without bevacizumab for the adjuvant treatment of patients with stage II or III colon cancer. J Clin Oncol 27(20):3385–3390. doi:10.1200/JCO.2009.21.9220
Saltz LB, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F, Couture F, Sirzen F, Cassidy J (2008) Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 26(12):2013–2019. doi:10.1200/jco.2007.14.9930
Giantonio BJ, Catalano PJ, Meropol NJ, O’Dwyer PJ, Mitchell EP, Alberts SR, Schwartz MA, Benson AB 3rd (2007) Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol 25(12):1539–1544. doi:10.1200/jco.2006.09.6305
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350(23):2335–2342. doi:10.1056/NEJMoa032691
Tewari KS, Sill M, Long HJ, Ramondetta LM, Landrum LM, Oaknin A, Reid TJ, Leitao MM, Michael HE, Monk BJ (2013) Incorporation of bevacizumab in the treatment of recurrent and metastatic cervical cancer: a phase III randomized trial of the Gynecologic Oncology Group. J Clin Oncol 31[Suppl] abstr 3
Pujade-Lauraine E, Hilpert F, Weber B, Reuss A, Poveda A, Kristensen G, Sorio R, Vergote IB, Witteveen P, Bamias A, Pereira D, Wimberger P, Oaknin A, Mirza MR, Follana P, Bollag DT, Ray-Coquard I, Investigators A (2012) AURELIA: a randomized phase III trial evaluating bevacizumab (BEV) plus chemotherapy (CT) for platinum (PT)-resistant recurrent ovarian cancer (OC). J Clin Oncol 30[Suppl] abstr LBA5002
Gianni L, Romieu GH, Lichinitser M, Serrano SV, Mansutti M, Pivot X, Mariani P, Andre F, Chan A, Lipatov O, Chan S, Wardley A, Greil R, Moore N, Prot S, Pallaud C, Semiglazov V (2013) AVEREL: a randomized phase III Trial evaluating bevacizumab in combination with docetaxel and trastuzumab as first-line therapy for HER2-positive locally recurrent/metastatic breast cancer. J Clin Oncol 31(14):1719–1725. doi:10.1200/JCO.2012.44.7912
Robert NJ, Dieras V, Glaspy J, Brufsky AM, Bondarenko I, Lipatov ON, Perez EA, Yardley DA, Chan SY, Zhou X, Phan SC, O’Shaughnessy J (2011) RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol 29(10):1252–1260. doi:10.1200/JCO.2010.28.0982
Brufsky AM, Hurvitz S, Perez E, Swamy R, Valero V, O’Neill V, Rugo HS (2011) RIBBON-2: a randomized, double-blind, placebo-controlled, phase III trial evaluating the efficacy and safety of bevacizumab in combination with chemotherapy for second-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol 29(32):4286–4293. doi:10.1200/jco.2010.34.1255
Miles DW, Chan A, Dirix LY, Cortes J, Pivot X, Tomczak P, Delozier T, Sohn JH, Provencher L, Puglisi F, Harbeck N, Steger GG, Schneeweiss A, Wardley AM, Chlistalla A, Romieu G (2010) Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol 28(20):3239–3247. doi:10.1200/jco.2008.21.6457
Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, Shenkier T, Cella D, Davidson NE (2007) Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med 357(26):2666–2676. doi:10.1056/NEJMoa072113
Miller KD, Chap LI, Holmes FA, Cobleigh MA, Marcom PK, Fehrenbacher L, Dickler M, Overmoyer BA, Reimann JD, Sing AP, Langmuir V, Rugo HS (2005) Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol 23(4):792–799. doi:10.1200/jco.2005.05.098
Spigel DR, Townley PM, Waterhouse DM, Fang L, Adiguzel I, Huang JE, Karlin DA, Faoro L, Scappaticci FA, Socinski MA (2011) Randomized phase II study of bevacizumab in combination with chemotherapy in previously untreated extensive-stage small-cell lung cancer: results from the SALUTE trial. J Clin Oncol 29(16):2215–2222. doi:10.1200/JCO.2010.29.3423
Reck M, von Pawel J, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V, Leighl N, Mezger J, Archer V, Moore N, Manegold C (2009) Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol 27(8):1227–1234. doi:10.1200/jco.2007.14.5466
Herbst RS, O’Neill VJ, Fehrenbacher L, Belani CP, Bonomi PD, Hart L, Melnyk O, Ramies D, Lin M, Sandler A (2007) Phase II study of efficacy and safety of bevacizumab in combination with chemotherapy or erlotinib compared with chemotherapy alone for treatment of recurrent or refractory non small-cell lung cancer. J Clin Oncol 25(30):4743–4750. doi:10.1200/JCO.2007.12.3026
Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH (2006) Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 355(24):2542–2550. doi:10.1056/NEJMoa061884
Rini BI, Halabi S, Rosenberg JE, Stadler WM, Vaena DA, Archer L, Atkins JN, Picus J, Czaykowski P, Dutcher J, Small EJ (2010) Phase III trial of bevacizumab plus interferon alfa versus interferon alfa monotherapy in patients with metastatic renal cell carcinoma: final results of CALGB 90206. J Clin Oncol 28(13):2137–2143. doi:10.1200/jco.2009.26.5561
Escudier B, Pluzanska A, Koralewski P, Ravaud A, Bracarda S, Szczylik C, Chevreau C, Filipek M, Melichar B, Bajetta E, Gorbunova V, Bay JO, Bodrogi I, Jagiello-Gruszfeld A, Moore N (2007) Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet 370(9605):2103–2111. doi:10.1016/s0140-6736(07)61904-7
Kindler HL, Niedzwiecki D, Hollis D, Sutherland S, Schrag D, Hurwitz H, Innocenti F, Mulcahy MF, O’Reilly E, Wozniak TF, Picus J, Bhargava P, Mayer RJ, Schilsky RL, Goldberg RM (2010) Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J Clin Oncol 28(22):3617–3622. doi:10.1200/jco.2010.28.1386
White D, Kassim A, Bhaskar B, Yi J, Wamstad K, Paton VE (2013) Results from AMBER, a randomized phase 2 study of bevacizumab and bortezomib versus bortezomib in relapsed or refractory multiple myeloma. Cancer 119(2):339–347. doi:10.1002/cncr.27745
Kindler HL, Karrison TG, Gandara DR, Lu C, Krug LM, Stevenson JP, Janne PA, Quinn DI, Koczywas MN, Brahmer JR, Albain KS, Taber DA, Armato SG 3rd, Vogelzang NJ, Chen HX, Stadler WM, Vokes EE (2012) Multicenter, double-blind, placebo-controlled, randomized phase II trial of gemcitabine/cisplatin plus bevacizumab or placebo in patients with malignant mesothelioma. J Clin Oncol 30(20):2509–2515. doi:10.1200/JCO.2011.41.5869
Kim KB, Sosman JA, Fruehauf JP, Linette GP, Markovic SN, McDermott DF, Weber JS, Nguyen H, Cheverton P, Chen D, Peterson AC, Carson WE 3rd, O’Day SJ (2012) BEAM: a randomized phase II study evaluating the activity of bevacizumab in combination with carboplatin plus paclitaxel in patients with previously untreated advanced melanoma. J Clin Oncol 30(1):34–41. doi:10.1200/jco.2011.34.6270
Sfakianos GP, Numnum TM, Halverson CB, Panjeti D, Kendrick JE 4th, Straughn JM Jr (2009) The risk of gastrointestinal perforation and/or fistula in patients with recurrent ovarian cancer receiving bevacizumab compared to standard chemotherapy: a retrospective cohort study. Gynecol Oncol 114(3):424–426. doi:10.1016/j.ygyno.2009.05.031
Han ES, Monk BJ (2007) What is the risk of bowel perforation associated with bevacizumab therapy in ovarian cancer? Gynecol Oncol 105(1):3–6. doi:10.1016/j.ygyno.2007.01.038
Yamada T, Kanazawa Y, Yokoi K, Uchida E (2013) A case of gastric cancer with perforation caused by chemotherapy with docetaxel and s-1. J Nippon Med School Nippon Ika Daigaku Zasshi 80(6):451–455
Park SR, Kim HK, Kim CG, Choi IJ, Lee JS, Lee JH, Ryu KW, Kim YW, Bae JM, Kim NK (2008) Phase I/II study of S-1 combined with weekly docetaxel in patients with metastatic gastric carcinoma. Br J Cancer 98(8):1305–1311. doi:10.1038/sj.bjc.6604312
Pear BL (1998) Pneumatosis intestinalis: a review. Radiology 207(1):13–19. doi:10.1148/radiology.207.1.9530294
Funding
None.
Conflict of interest
None..
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Figure 1
Meta-regression analysis of trends between treatment duration and relative risk of GI perforation, Symbols Each study is represented by a circle, the diameter of which is proportional to its statistical weight (JPEG 358 kb)
Supplemental Figure 2
Funnel plot of standard error by log-relative risk for GI perforation. (JPEG 209 kb)
Rights and permissions
About this article
Cite this article
Qi, WX., Shen, Z., Tang, LN. et al. Bevacizumab increases the risk of gastrointestinal perforation in cancer patients: a meta-analysis with a focus on different subgroups. Eur J Clin Pharmacol 70, 893–906 (2014). https://doi.org/10.1007/s00228-014-1687-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-014-1687-9