Abstract
Background and objectives:
A high inter-individual variability in the pharmacokinetics of lamotrigine has been observed. Lamotrigine is primarily metabolized by UGT1A4 and UGT2B7, both of which show genetic polymorphisms. Both genetic and non-genetic factors may influence the pharmacokinetics of lamotrigine. The aim of this study was to determine the pharmacokinetic parameters of lamotrigine and to investigate the effect of genetic variants of UGT1A4 and UGT2B7 and various non-genetic factors on its pharmacokinetics.
Methods:
Four single nucleotide polymorphisms (SNPs; UGT1A4 142 T>G, UGT1A4 70C>T, UGT2B7 372A>G, UGT2B7 -161C>T) were identified using the TaqMan Allelic Discrimination Assay. Data were analyzed using NONMEM. Model evaluation was performed using the bootstrap approach and predictive check.
Results:
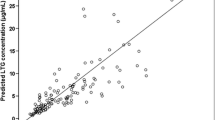
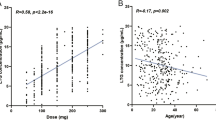
A total of 116 samples were obtained from 75 patients and included in the population analysis. The use of enzyme inducers, valproic acid, and the UGT2B7-161 C>T SNP were found to significantly influence lamotrigine apparent clearance (CL/F). Lamotrigine CL/F in patients carrying the UGT2B7 -161 CT or TT SNP was 18% lower than that in patients carrying the UGT2B7 -161 CC SNP.
Conclusion:
Both genetic and non-genetic factors were found to influence lamotrigine pharmacokinetics. These factors should be considered when determining lamotrigine dosing. The model presented here could be useful for lamotrigine dose adjustment in clinical practice.

Similar content being viewed by others
References
Johannessen SI, Tomson T (2006) Pharmacokinetic variability of newer antiepileptic drugs: when is monitoring needed? Clin Pharmacokinet 45:1061–1075
Milovanovic JR, Jankovic SM (2009) Population pharmacokinetics of lamotrigine in patients with epilepsy. Int J Clin Pharmacol Ther 47:752–760
Punyawudho B, Ramsay RE, Macias FM, Rowan AJ, Collins JF, Brundage RC, Birnbaum AK (2008) Population pharmacokinetics of lamotrigine in elderly patients. J Clin Pharmacol 48:455–463
Rivas N, Buelga DS, Elger CE, Santos-Borbujo J, Otero MJ, Dominguez-Gil A, Garcia MJ (2008) Population pharmacokinetics of lamotrigine with data from therapeutic drug monitoring in German and Spanish patients with epilepsy. Ther Drug Monit 30:483–489
Grasela TH, Fiedler-Kelly J, Cox E, Womble GP, Risner ME, Chen C (1999) Population pharmacokinetics of lamotrigine adjunctive therapy in adults with epilepsy. J Clin Pharmacol 39:373–384
Hussein Z, Posner J (1997) Population pharmacokinetics of lamotrigine monotherapy in patients with epilepsy: retrospective analysis of routine monitoring data. Br J Clin Pharmacol 43:457–465
Miners JO, McKinnon RA, Mackenzie PI (2002) Genetic polymorphisms of UDP-glucuronosyltransferases and their functional significance. Toxicology 181–182:453–456
Mori A, Maruo Y, Iwai M, Sato H, Takeuchi Y (2005) UDP-glucuronosyltransferase 1A4 polymorphisms in a Japanese population and kinetics of clozapine glucuronidation. Drug Metab Dispos 33:672–675
Coffman BL, King CD, Rios GR, Tephly TR (1998) The glucuronidation of opioids, other xenobiotics, and androgens by human UGT2B7Y(268) and UGT2B7H(268). Drug Metab Dispos 26:73–77
Coffman BL, Rios GR, King CD, Tephly TR (1997) Human UGT2B7 catalyzes morphine glucuronidation. Drug Metab Dispos 25:1–4
Chung JY, Cho JY, Yu KS, Kim JR, Lim KS, Sohn DR, Shin SG, Jang IJ (2008) Pharmacokinetic and pharmacodynamic interaction of lorazepam and valproic acid in relation to UGT2B7 genetic polymorphism in healthy subjects. Clin Pharmacol Ther 83:595–600
Rowland A, Elliot DJ, Williams JA, Mackenzie PI, Dickinson RG, Miners JO (2006) In vitro characterization of lamotrigine N2-glucuronidation and the lamotrigine-valproic acid interaction. Drug Metab Dispos 34:1055–1062
Bhasker CR, McKinnon W, Stone A, Lo AC, Kubota T, Ishizaki T, Miners JO (2000) Genetic polymorphism of UDP-glucuronosyltransferase 2B7 (UGT2B7) at amino acid 268: ethnic diversity of alleles and potential clinical significance. Pharmacogenetics 10:679–685
Fisher MB, Vandenbranden M, Findlay K, Burchell B, Thummel KE, Hall SD, Wrighton SA (2000) Tissue distribution and interindividual variation in human UDP-glucuronosyltransferase activity: relationship between UGT1A1 promoter genotype and variability in a liver bank. Pharmacogenetics 10:727–739
Innocenti F, Liu W, Fackenthal D, Ramirez J, Chen P, Ye X, Wu X, Zhang W, Mirkov S, Das S, Cook E Jr, Ratain MJ (2008) Single nucleotide polymorphism discovery and functional assessment of variation in the UDP-glucuronosyltransferase 2B7 gene. Pharmacogenet Genomics 18:683–697
Saeki M, Saito Y, Jinno H, Tanaka-Kagawa T, Ohno A, Ozawa S, Ueno K, Kamakura S, Kamatani N, Komamura K, Kitakaze M, Sawada J (2004) Single nucleotide polymorphisms and haplotype frequencies of UGT2B4 and UGT2B7 in a Japanese population. Drug Metab Dispos 32:1048–1054
Gulcebi MI, Ozkaynakci A, Goren MZ, Aker RG, Ozkara C, Onat FY (2011) The relationship between UGT1A4 polymorphism and serum concentration of lamotrigine in patients with epilepsy. Epilepsy Res 95:1–8
Blanca Sanchez M, Herranz JL, Leno C, Arteaga R, Oterino A, Valdizan EM, Nicolas JM, Adin J, Shushtarian M, Armijo JA (2010) UGT2B7_-161C > T polymorphism is associated with lamotrigine concentration-to-dose ratio in a multivariate study. Ther Drug Monit 32:177–184
Ramsay RE, Pellock JM, Garnett WR, Sanchez RM, Valakas AM, Wargin WA, Lai AA, Hubbell J, Chern WH, Allsup T et al (1991) Pharmacokinetics and safety of lamotrigine (Lamictal) in patients with epilepsy. Epilepsy Res 10:191–200
Beal SL, Sheiner LB, Boeckmann AJE (1989–2006) NONMEM users guides. University of California, San Francisco
Jonsson EN, Karlsson MO (1999) Xpose—an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Methods Programs Biomed 58:51–64
Jadhav PR, Gobburu JV (2005) A new equivalence based metric for predictive check to qualify mixed-effects models. AAPS J 7:E523–E531
Yano Y, Beal SL, Sheiner LB (2001) Evaluating pharmacokinetic/pharmacodynamic models using the posterior predictive check. J Pharmacokinet Pharmacodyn 28:171–192
Chan V, Morris RG, Ilett KF, Tett SE (2001) Population pharmacokinetics of lamotrigine. Ther Drug Monit 23:630–635
Patsalos PN, Froscher W, Pisani F, van Rijn CM (2002) The importance of drug interactions in epilepsy therapy. Epilepsia 43:365–385
Christensen J, Petrenaite V, Atterman J, Sidenius P, Ohman I, Tomson T, Sabers A (2007) Oral contraceptives induce lamotrigine metabolism: evidence from a double-blind, placebo-controlled trial. Epilepsia 48:484–489
Lalic M, Cvejic J, Popovic J, Bozic K, Golocorbin-Kon S, Al-Salami H, Mikov M (2009) Lamotrigine and valproate pharmacokinetics interactions in epileptic patients. Eur J Drug Metab Pharmacokinet 34:93–99
Yafune A, Ishiguro M (1999) Bootstrap approach for constructing confidence intervals for population pharmacokinetic parameters. I: A use of bootstrap standard error. Stat Med 18:581–599
Booth BP, Gobburu JV (2003) Considerations in analyzing single-trough concentrations using mixed-effects modeling. J Clin Pharmacol 43:1307–1315
Acknowledgments
This work was supported by the Thailand Research Fund (MRG-5480122), the 90th Anniversary of Chulalongkorn University Fund (Ratchadaphiseksomphot Endowment Fund), and the Faculty of Pharmacy Research Fund. The authors would like to thank all of the staffs of the epilepsy and psychiatric outpatient clinics, Prasat Neurological Institute. We also thank Dr. Pornanong Aramwit for her invaluable comments and great assistance. We are grateful to all the participants for their contributions to this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Part of this work was presented at the World Conference on Pharmacometrics (WCoP) 2012, Seoul, Korea.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 473 kb)
Rights and permissions
About this article
Cite this article
Singkham, N., Towanabut, S., Lertkachatarn, S. et al. Influence of the UGT2B7 -161C>T polymorphism on the population pharmacokinetics of lamotrigine in Thai patients. Eur J Clin Pharmacol 69, 1285–1291 (2013). https://doi.org/10.1007/s00228-012-1449-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-012-1449-5