Abstract
Objectives
To compare the systemic exposure for intranasal mometasone furoate (MF) and fluticasone propionate (FP) aqueous nasal sprays (ANS) in terms of serum and urinary cortisol parameters and plasma pharmacokinetics.
Methods
Twelve healthy subjects completed this three-way, cross-over study. They received FPANS (50 μg/spray), MFANS (50 μg/spray) or placebo ANS, eight sprays per nostril every 8 h for 4 days. Cortisol measurements were made at baseline and day 4. FP and MF plasma concentrations were also measured on day 4.
Results
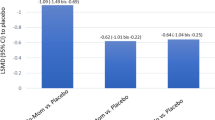
MFANS produced similar mean plasma AUC (123 pmol/l h) to FPANS (112 pmol/l h). Despite the use of high doses, necessary to generate adequate pharmacokinetic data, only minor reductions in cortisol parameters were found, with no difference between FPANS and MFANS.
Conclusions
FP and MF have similar and very low systemic bioavailability when administered intranasally using a high-dose regimen. It is therefore unlikely that therapeutic doses of intranasal FP or MF will produce dissimilar or significant degrees of systemic exposure or systemic effects.

Similar content being viewed by others
References
Dolovich J, O’Connor M, Stepner N, Smith A, Sharma RK (1994) Double-blind comparison of intranasal fluticasone propionate, 200 micrograms, once daily with 200 micrograms twice daily in the treatment of patients with severe seasonal allergic rhinitis to ragweed. Ann Allergy 72:435–440
Pedersen B, Dahl R, Richards DH (1995) Once daily fluticasone propionate aqueous nasal spray controls symptoms of most patients with seasonal allergic rhinitis. Allergy 50:794–799
Hebert JR, Nolop K, Lutsky BN (1996) Once-daily mometasone furoate aqueous nasal spray (Nasonex) in seasonal allergic rhinitis: an active- and placebo-controlled study. Allergy 51:569–576
Drouin M, Yang WH, Bertrand B (1996) Once daily mometasone furoate aqueous nasal spray is as effective as twice daily beclomethasone dipropionate for treating perennial allergic rhinitis patients. Ann Allergy Asthma Immunol 77:153–160
Daley-Yates PT, Baker R (2001) Systemic bioavailability of fluticasone propionate administered as nasal drops and the aqueous nasal spray formulations. Br J Clin Pharmacol 51:103–105
McDowell JE, Mackie AE, Ventresca GP, Bye A (1997) Pharmacokinetics and bioavailability of intranasal fluticasone in humans. Clin Drug Invest 1:44–52
Schering-Plough Research Institute (1998) SCH 32088: single dose absolute bioavailability study of mometasone nasal spray in volunteers with evidence of allergic rhinitis. Study No. C93–196 (data on file)
Callejas S, Biddlecombe R, Jones A, Joyce K, Pereira A, Pleasance S (1998) Determination of the glucocorticoid fluticasone propionate in plasma by automated solid-phase extraction and liquid chromatography-tandem mass spectrometry. J Chromatogr 718:243–250
FDA Summary Basis of Approval (2000) NDA 20762, Nasonex Nasal Spray Medical Officer Review
Price AC, Daley-Yates PT, Wright AM, Callejas S (2000) Negligible absolute bioavailability and no HPA-axis effects after multiple 200 µg daily doses of Fluticasone propionate (FP) administered from the aqueous nasal spray (FPANS). Eur Resp J 16:279s
Daley-Yates PT, Price AC, Sisson JR, Pereira A, Dallow N (2001) Beclomethasone dipropionate: absolute bioavailability, pharmacokinetics and metabolism following intravenous, oral, intranasal and inhaled administration in man. Br J Clin Pharmacol 51:400–409
Jeal W, Faulds D (1997) Triamcinolone acetonide. A review of its pharmacological properties and therapeutic efficacy in the management of allergic rhinitis. Drugs 53:257–280
Thorsson L, Borga O, Edsbaker S (1999) Systemic availability of budesonide after nasal administration of three different formulations: pressurized aerosol, aqueous pump spray and powder. Br J Clin Pharmacol 47:619–624
Argenti D, Colligon I, Heald D (1994) Nasal mucosal inflammation has no effect on the absorption of intranasal triamcinolone acetonide. J Clin Pharmacol 34:854–858
Vargas R, Dockhorn RJ, Findlay SR, Korenblat PE, Field EA, Kral KM (1998) Effect of fluticasone propionate aqueous nasal spray versus oral prednisone on the hypothalamic-pituitary-adrenal axis. J Allergy Clin Immunol 102:191–197
Van As A, Bronsky E, Grossman J, Meltzer E, Ratner P, Reed C (1991) Dose tolerance study of fluticasone propionate aqueous nasal spray in patients with seasonal allergic rhinitis. Ann Allergy 67:156–162
Brannan MD, Seiberling M, Cutler DL (1996) Lack of systemic activity with intranasal mometasone furoate. J Allergy Clin Immunol 97:198
Brannan MD, Herron JM, Reidenberg P (1996) Lack of HPA axis suppression following 36 days of intranasal mometasone furoate. Ann Allergy Asthma Immunol 78:154
Crim C, Pierre LN, Daley-Yates PT (2001) A review of the pharmacology and pharmacokinetics of fluticasone propionate and mometosone furoate. Clin Therapeut 23:1339–1354
Daley-Yates PT, Richards DH (2001) Pharmacokinetic and pharmacodynamic relationships for intranasal corticosteroids (INCS). J Allergy Clin Immunol 107:S313
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Daley-Yates, P.T., Kunka, R.L., Yin, Y. et al. Bioavailability of fluticasone propionate and mometasone furoate aqueous nasal sprays. Eur J Clin Pharmacol 60, 265–268 (2004). https://doi.org/10.1007/s00228-004-0763-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-004-0763-y