Abstract
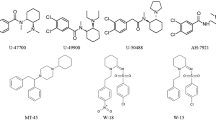
A novel platform is introduced for simultaneous identification and quantification of new psychoactive substances (NPS) in blood matrix, without the necessity of using authentic reference standards. The instrumentation consisted of gas chromatography (GC) coupled to nitrogen chemiluminescence detection (NCD) and atmospheric pressure chemical ionization quadrupole time-of-flight mass spectrometry (APCI-QTOFMS). In this concept, the GC flow is divided in appropriate proportions between NCD for single-calibrant quantification, utilizing the detector’s equimolar response to nitrogen, and QTOFMS for accurate mass-based identification. The principle was proven by analyzing five NPS, bupropion, desoxypipradrol (2-DPMP), mephedrone, methylone, and naphyrone, in sheep blood. The samples were spiked with the analytes post-extraction to avoid recovery considerations at this point. All the NPS studies produced a protonated molecule in APCI resulting in predictable fragmentation with high mass accuracy. The N-equimolarity of quantification by NCD was investigated by using external calibration with the secondary standard caffeine at five concentration levels between 0.17 and 1.7 mg/L in blood matrix as five replicates. The equimolarity was on average 98.7 %, and the range of individual equimolarity determinations was 76.7–130.1 %. The current analysis platform affords a promising approach to instant simultaneous qualitative and quantitative analysis of drugs in the absence of authentic reference standards, not only in forensic and clinical toxicology but also in other bioanalytical applications.

Analytical & Bioanalytical Chemistry

Similar content being viewed by others
References
Ojanperä I, Kolmonen M, Pelander A. Current use of high-resolution mass spectrometry in drug screening relevant to clinical and forensic toxicology and doping control. Anal Bioanal Chem. 2012;403:1203–20.
Arnhard K, Gottschall A, Pitterl F, Oberacher H. Applying ‘Sequential windowed acquisition of all theoretical fragment Ion mass spectra’ (SWATH) for systematic toxicological analysis with liquid chromatography-high-resolution tandem mass spectrometry. Anal Bioanal Chem. 2015;407:405–14.
Nedderman AN, Dear GJ, North S, Obach RS, Higton D. From definition to implementation: a cross-industry perspective of past, current and future MIST strategies. Xenobiotica. 2011;41:605–22.
Laks S, Pelander A, Vuori E, Ali-Tolppa E, Sippola E, Ojanperä I. Analysis of street drugs in seized material without primary reference standards. Anal Chem. 2004;76:7375–9.
Rasanen I, Kyber M, Szilvay I, Rintatalo J, Ojanperä I. Straightforward single-calibrant quantification of seized designer drugs by liquid chromatography-chemiluminescence nitrogen detection. Forensic Sci Int. 2014;237:119–25.
Ojanperä S, Tuominen S, Ojanperä I. Single-calibrant quantification of drugs in plasma and whole blood by liquid chromatography-chemiluminescence nitrogen detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;856:239–44.
Ojanperä S, Rasanen I, Sistonen J, Pelander A, Vuori E, Ojanperä I. Quantification of drugs in plasma without primary reference standards by liquid chromatography-chemiluminescence nitrogen detection: application to tramadol metabolite ratios. Ther Drug Monit. 2007;29:423–8.
Horai H, Arita M, Kanaya S, Nihei Y, Ikeda T, Suwa K, et al. MassBank: a public repository for sharing mass spectral data for life sciences. J Mass Spectrom. 2010;45:703–14.
Strano Rossi S, Odoardi S, Gregori A, Peluso G, Ripani L, Ortar G, et al. An analytical approach to the forensic identification of different classes of new psychoactive substances (NPSs) in seized materials. Rapid Commun Mass Spectrom. 2014;28:1904–16.
Tyrkkö E, Pelander A, Ketola RA, Ojanperä I. In silico and in vitro metabolism studies support identification of designer drugs in human urine by liquid chromatography/quadrupole-time-of-flight mass spectrometry. Anal Bioanal Chem. 2013;405:6697–709.
Meyer MR, Prosser D, Maurer HH. Studies on the metabolism and detectability of the designer drug β-naphyrone in rat urine using GC-MS and LC-HR-MS/MS. Drug Test Anal. 2013;5:259–65.
Wachsmuth CJ, Hahn TA, Oefner PJ, Dettmer K. Enhanced metabolite profiling using a redesigned atmospheric pressure chemical ionization source for gas chromatography coupled to high-resolution time-of-flight mass spectrometry. Anal Bioanal Chem. 2015;407:6669–80.
Pacchiarotta T, Derks RJ, Hurtado-Fernandez E, van Bezooijen P, Henneman A, Schiewek R, et al. Online spectral library for GC-atmospheric pressure chemical ionization-ToF MS. Bioanalysis. 2013;5:1515–25.
Adamowicz P, Gieroń J, Gil D, Lechowicz W, Skulska A, Tokarczyk B. The prevalence of new psychoactive substances in biological material—a three-year review of casework in Poland. Drug Test Anal. 2016;8:64–71.
Elliott S, Evans J. A 3-year review of new psychoactive substances in casework. Forensic Sci Int. 2014;243:55–60.
Hajkova K, Jurasek B, Sykora D, Palenicek T, Miksatkova P, Kuchar M. Salting-out-assisted liquid-liquid extraction as a suitable approach for determination of methoxetamine in large sets of tissue samples. Anal Bioanal Chem. 2016;408:1171–81.
Adam F, Bertoncini F, Brodusch N, Durand E, Thiébaut D, Espinat D, et al. New benchmark for basic and neutral nitrogen compounds speciation in middle distillates using comprehensive two-dimensional gas chromatography. J Chromatogr A. 2007;1148:55–64.
Dijkmans T, Djokic M, Van Geem K, Marin G. Comprehensive compositional analysis of sulfur and nitrogen containing compounds in shale oil using GC x GC - FID/SCD/NCD/TOF-MS. Fuel. 2015;140:398–406.
Özel MZ, Hamilton JF, Lewis AC. New sensitive and quantitative analysis method for organic nitrogen compounds in urban aerosol samples. Environ Sci Technol. 2011;45:1497–505.
Kocak D, Özel MZ, Gogus F, Hamilton JF, Lewis AC. Determination of volatile nitrosamines in grilled sheep and vegetables using comprehensive gas chromatography-nitrogen chemiluminescence detection. Food Chem. 2012;135:2215–20.
Dahal UP, Jones JP, Davis JA, Rock DA. Small molecule quantification by liquid chromatography-mass spectrometry for metabolites of drugs and drug candidates. Drug Metab Dispos. 2011;39:2355–60.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The experiments were conducted in accordance with animal ethical care.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ojanperä, I., Mesihää, S., Rasanen, I. et al. Simultaneous identification and quantification of new psychoactive substances in blood by GC-APCI-QTOFMS coupled to nitrogen chemiluminescence detection without authentic reference standards. Anal Bioanal Chem 408, 3395–3400 (2016). https://doi.org/10.1007/s00216-016-9461-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9461-8