Abstract
Summary
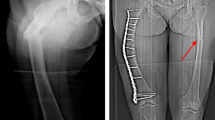
In this report, we present three cases of individuals from the same family with a diagnosis of CMT with severe tibia bone microarchitecture deterioration assessed by HR-pQCT. Charcot-Marie-Tooth disease (CMT) or hereditary neuropathy involves both motor and sensory nerves. Falls are often the first manifestation in these patients and represent an important risk factor for fracture. The reduction of mechanical input on bone inhibits bone formation by osteoblasts and accelerates bone resorption by osteoclasts, leading to disuse osteoporosis. We report three cases of individuals from the same family with a diagnosis of CMT with severe tibia bone microarchitecture deterioration assessed by high-resolution peripheral quantitative computed tomography (HR-pQCT). This affectation was exclusive to the tibia; the radius remained undamaged, showing the consequences of the lack of mobility and mechanical stimulation. Physical activity and rehabilitation, in addition to adequate calcium and vitamin D supplementation, may play an essential role in the management of this disease.

Similar content being viewed by others
References
Szigeti K, Lupski JR (2009) Charcot-Marie-Tooth disease. Eur J Hum Genet 17(6):703–710
Hoyle JC, Isfort MC, Roggenbuck J, Arnold WD (2015) The genetics of Charcot-Marie-Tooth disease: current trends and future implications for diagnosis and management. Appl Clin Genet 8:235–243
Louwerens JWK (2018) Operative treatment algorithm for foot deformities in Charcot-Marie-Tooth disease. Algorithmus für die operative Behandlung von Fußdeformitäten bei der Charcot-Marie-Tooth-Krankheit. Oper Orthop Traumatol 30(2):130–146
Timmerman V, Strickland AV, Züchner S (2014) Genetics of Charcot-Marie-Tooth (CMT) disease within the frame of the human genome project success. Genes (Basel) 5(1):13–32
Pouwels S, de Boer A, Leufkens HG, Weber WE, Cooper C, de Vries F (2014) Risk of fracture in patients with Charcot-Marie-Tooth disease. Muscle Nerve 50(6):919–924
Hsu JD (1979) Extremity fractures in children with neuromuscular disease. Johns Hopkins Med J 145(3):89–93
Aujla RS, Gulihar A, Taylor GJ (2008) Acromial stress fracture in a young wheelchair user with Charcot-Marie-Tooth disease: a case report. Cases J 1(1):359
Quintart C, Baillon JM, Libotte M (1999) Fracture pathologique du tibia compliquant une maladie de Charcot-Marie-Tooth [Pathologic fracture of the tibia associated with Charcot-Marie-Tooth disease]. Acta Orthop Belg 65(1):105–108
Garland DE, Stewart CA, Adkins RH, Hu SS, Rosen C, Liotta FJ, Weinstein DA (1992) Osteoporosis after spinal cord injury. J Orthop Res 10(3):371–378
Giannotti S, Bottai V, Dell’osso G et al (2013) Disuse osteoporosis of the upper limb: assessment of thirty patients. Clin Cases Miner Bone Metab 10(2):129–132
Robling AG, Castillo AB, Turner CH (2006) Biomechanical and molecular regulation of bone remodeling. Annu Rev Biomed Eng 8(1):455–449
MacNeil JA, Boyd SK (2008) Improved reproducibility of high-resolution peripheral quantitative computed tomography for measurement of bone quality. Med Eng Phys 30:792–799
Nishiyama KK, Shane E (2013) Clinical imaging of bone microarchitecture with HR-pQCT. Curr Osteoporos Rep 11(2):147–155
Boutroy S, Bouxsein ML, Munoz F, Delmas PD (2005) In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab 90(12):6508–6515
Guðmundsson B, Ólafsson E, Jakobsson F, Lúðvígsson P (2010) Prevalence of symptomatic Charcot-Marie-Tooth disease in Iceland: a study of a well-defined population. Neuroepidemiology 34:13–17
Palau F, Cuesta A, Pedrola L (2002) Avances en la genética molecular de las neuropatías hereditarias. Rev Neurol 35:246–253
Boutroy S, Van Rietbergen B, Sornay-Rendu E et al (2008) Finite element analysis based on in vivo hr-pqct images of the distal radius is associated with wrist fracture in postmenopausal women. J Bone Miner Res 23:392–399
Burghardt AJ, Link TM, Majumdar S (2011) High-resolution computed tomography for clinical imaging of bone microarchitecture. Clin Orthop Relat Res 469:2179–2193
Balonga MC, Zanchetta MB (2013) Grave deterioro de la microarquitectura ósea inducido por desuso y denervacion crónica. Actual Osteol 9(2):205–206
Keyak JH, Koyama AK, LeBlanc A, Lu Y, Lang TF (2009) Reduction in proximal femoral strength due to long-duration spaceflight. Bone. 44(3):449–453
Kannus P, Järvinen M, Sievänen H, Oja P, Vuori I (1994) Osteoporosis in men with a history of tibial fracture. J Bone Miner Res 9(3):423–429
Kazakia GJ, Tjong W, Nirody JA, Burghardt AJ, Carballido-Gamio J, Patsch JM, Link T, Feeley BT, Benjamin Ma C (2014) The influence of disuse on bone microstructure and mechanics assessed by HR-pQCT. Bone. 63:132–140
Alexandre C, Vico L (2011) Pathophysiology of bone loss in disuse osteoporosis. Joint Bone Spine 78(6):572–576
Takata S, Yasui N (2001) Disuse Osteoporosis. J Med Investig 48(3–4):147–156
Bellido T (2014) Osteocyte-driven bone remodeling. Calcif Tissue Int 94(1):25–34
Young MJ, Marshall A, Adams JE, Selby PL, Boulton AJ (1995) Osteopenia, neurological dysfunction, and the development of Charcot neuroarthropathy. Diabetes Care 18(1):34–38
Zhang K, Barragan-Adjemian C, Ye L, Kotha S, Dallas M, Lu Y, Zhao S, Harris M, Harris SE, Feng JQ, Bonewald LF (2006) E11/gp38 selective expression in osteocytes: regulation by mechanical strain and role in dendrite elongation. Mol Cell Biol 26(12):4539–4552
Winkler DG, Sutherland MK, Geoghegan JC et al (2003) Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J 22(23):6267–6276
Frost HM (1997) Defining osteopenias and osteoporoses: another view (with insights from a new paradigm). Bone. 20(5):385–391
Ferretti JL, Cointry GR, Capozza RF, Frost HM (2003) Bone mass, bone strength, muscle-bone interactions, osteopenias and osteoporoses. Mech Ageing Dev 124(3):269–279
Li CY, Price C, Delisser K, Nasser P, Laudier D, Clement M, Jepsen KJ, Schaffler MB (2005) Long-term disuse osteoporosis seems less sensitive to bisphosphonate treatment than other osteoporosis. J Bone Miner Res 20(1):117–124
Milsom S, Lin S-Y(S), Cornish J, Sharma S (2016) Disuse osteoporosis: a better understanding of pathophysiology may lead to potential therapies. J Diabetol Endocrinol 1(1):1–4
Kutilek S (2017) Denosumab treatment of severe disuse osteoporosis in a boy with spinal muscular atrophy. Acta Med Iran 55(10):658–660
Daly RM, Dalla Via J, Duckham RL, Fraser SF, Helge EW (2019) Exercise for the prevention of osteoporosis in postmenopausal women: an evidence-based guide to the optimal prescription. Braz J Phys Ther 23(2):170–180
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 24 kb)
Rights and permissions
About this article
Cite this article
Abdala, R., Levi, L., Longobardi, V. et al. Severe bone microarchitecture deterioration in a family with hereditary neuropathy: evidence of the key role of the mechanostat. Osteoporos Int 31, 2477–2480 (2020). https://doi.org/10.1007/s00198-020-05674-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-020-05674-9