Abstract
Summary
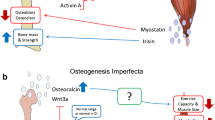
Mice with osteogenesis imperfecta (+/oim), a disorder of bone fragility, were bred to mice with muscle over growth to test whether increasing muscle mass genetically would improve bone quality and strength. The results demonstrate that femora from mice carrying both mutations have greater mechanical integrity than their +/oim littermates.
Introduction
Osteogenesis imperfecta is a heritable connective tissue disorder due primarily to mutations in the type I collagen genes resulting in skeletal deformity and fragility. Currently, there is no cure, and therapeutic strategies encompass the use of antiresorptive pharmaceuticals and surgical bracing, with limited success and significant potential for adverse effects. Bone, a mechanosensing organ, can respond to high mechanical loads by increasing new bone formation and altering bone geometry to withstand increased forces. Skeletal muscle is a major source of physiological loading on bone, and bone strength is proportional to muscle mass.
Methods
To test the hypothesis that congenic increases in muscle mass in the osteogenesis imperfecta murine model mouse (oim) will improve their compromised bone quality and strength, heterozygous (+/oim) mice were bred to mice deficient in myostatin (+/mstn), a negative regulator of muscle growth. The resulting adult offspring were evaluated for hindlimb muscle mass, and bone microarchitecture, physiochemistry, and biomechanical integrity.
Results
+/oim mice deficient in myostatin (+/mstn +/oim) were generated and demonstrated that myostatin deficiency increased body weight, muscle mass, and biomechanical strength in +/mstn +/oim mice as compared to +/oim mice. Additionally, myostatin deficiency altered the physiochemical properties of the +/oim bone but did not alter bone remodeling.
Conclusions
Myostatin deficiency partially improved the reduced femoral bone biomechanical strength of adult +/oim mice by increasing muscle mass with concomitant improvements in bone microarchitecture and physiochemical properties.



Similar content being viewed by others
References
Van Dijk FS, Sillence DO (2014) Osteogenesis imperfecta: clinical diagnosis, nomenclature and severity assessment. Am J Med Genet A 164:1470–1481
Cundy T (2012) Recent advances in osteogenesis imperfecta. Calcif Tissue Int 90:439–449
Veilleux LN, Lemay M, Pouliot-Laforte A, Cheung MS, Glorieux FH, Rauch F (2014) Muscle anatomy and dynamic muscle function in osteogenesis imperfecta type I. J Clin Endocrinol Metab 99:E356–E362
Chipman SD, Sweet HO, McBride DJ Jr, Davisson MT, Marks SC Jr, Shuldiner AR, Wenstrup RJ, Rowe DW, Shapiro JR (1993) Defective pro alpha 2(I) collagen synthesis in a recessive mutation in mice: a model of human osteogenesis imperfecta. Proc Natl Acad Sci U S A 90:1701–1705
McBride DJ Jr, Shapiro JR, Dunn MG (1998) Bone geometry and strength measurements in aging mice with the oim mutation. Calcif Tissue Int 62:172–176
Camacho NP, Hou L, Toledano TR, Ilg WA, Brayton CF, Raggio CL, Root L, Boskey AL (1999) The material basis for reduced mechanical properties in oim mice bones. J Bone Miner Res 14:264–272
Gentry BA, Ferreira JA, McCambridge AJ, Brown M, Phillips CL (2010) Skeletal muscle weakness in osteogenesis imperfecta mice. Matrix Biol 29:638–644
Saban J, Zussman MA, Havey R, Patwardhan AG, Schneider GB, King D (1996) Heterozygous oim mice exhibit a mild form of osteogenesis imperfecta. Bone 19:575–579
Yao X, Carleton SM, Kettle AD, Melander J, Phillips CL, Wang Y (2013) Gender-dependence of bone structure and properties in adult osteogenesis imperfecta murine model. Ann Biomed Eng 41:1139–1149
Carriero A, Zimmermann EA, Paluszny A, Tang SY, Bale H, Busse B, Alliston T, Kazakia G, Ritchie RO, Shefelbine SJ (2014) How tough is brittle bone? investigating osteogenesis imperfecta in mouse bone. J Bone Miner Res 29:1392–1401
Warden SJ, Hurst JA, Sanders MS, Turner CH, Burr DB, Li J (2005) Bone adaptation to a mechanical loading program significantly increases skeletal fatigue resistance. J Bone Miner Res 20:809–816
Turner CH (2006) Bone strength: current concepts. Ann N Y Acad Sci 1068:429–446
Kodama Y, Umemura Y, Nagasawa S, Beamer WG, Donahue LR, Rosen CR, Baylink DJ, Farley JR (2000) Exercise and mechanical loading increase periosteal bone formation and whole bone strength in C57BL/6J mice but not in C3H/Hej mice. Calcif Tissue Int 66:298–306
Hamrick MW, Samaddar T, Pennington C, McCormick J (2006) Increased muscle mass with myostatin deficiency improves gains in bone strength with exercise. J Bone Miner Res 21:477–483
Kollias HD, McDermott JC (2008) Transforming growth factor-beta and myostatin signaling in skeletal muscle. J Appl Physiol 104:579–587
McPherron AC, Lawler AM, Lee SJ (1997) Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 387:83–90
Gentry BA, Ferreira JA, Phillips CL, Brown M (2011) Hindlimb skeletal muscle function in myostatin-deficient mice. Muscle Nerve 43:49–57
Mosher DS, Quignon P, Bustamante CD, Sutter NB, Mellersh CS, Parker HG, Ostrander EA (2007) A mutation in the myostatin gene increases muscle mass and enhances racing performance in heterozygote dogs. PLoS Genet 3:e79
Elkasrawy MN, Hamrick MW (2010) Myostatin (GDF-8) as a key factor linking muscle mass and bone structure. J Musculoskelet Neuronal Interact 10:56–63
Beamer WG, Shultz KL, Donahue LR, Churchill GA, Sen S, Wergedal JR, Baylink DJ, Rosen CJ (2001) Quantitative trait loci for femoral and lumbar vertebral bone mineral density in C57BL/6J and C3H/HeJ inbred strains of mice. J Bone Miner Res 16:1195–1206
Carleton SM, McBride DJ, Carson WL, Huntington CE, Twenter KL, Rolwes KM, Winkelmann CT, Morris JS, Taylor JF, Phillips CL (2008) Role of genetic background in determining phenotypic severity throughout postnatal development and at peak bone mass in Col1a2 deficient mice (oim). Bone 42:681–694
Mandair GS, Morris MD (2015) Contributions of Raman spectroscopy to the understanding of bone strength. BoneKEy Rep 4
Stegemann H, Stalder K (1967) Determination of hydroxyproline. Clin Chim Acta 18:267–273
Naylor K, Eastell R (2012) Bone turnover markers: use in osteoporosis. Nat Rev Rheumatol 8:379–389
Lee SJ (2007) Quadrupling muscle mass in mice by targeting TGF-beta signaling pathways. PLoS One 2:e789
Hamrick MW, McPherron AC, Lovejoy CO (2002) Bone mineral content and density in the humerus of adult myostatin-deficient mice. Calcif Tissue Int 71:63–68
Hamrick MW (2003) Increased bone mineral density in the femora of GDF8 knockout mice. Anat Rec A: Discov Mol Cell Evol Biol 272:388–391
Zhang H, Doty SB, Hughes C, Dempster D, Camacho NP (2007) Increased resorptive activity and accompanying morphological alterations in osteoclasts derived from the oim/oim mouse model of osteogenesis imperfecta. J Cell Biochem 102:1011–1020
Uveges TE, Collin-Osdoby P, Cabral WA, Ledgard F, Goldberg L, Bergwitz C, Forlino A, Osdoby P, Gronowicz GA, Marini JC (2008) Cellular mechanism of decreased bone in Brtl mouse model of OI: imbalance of decreased osteoblast function and increased osteoclasts and their precursors. J Bone Miner Res 23:1983–1994
Chen F, Guo R, Itoh S et al (2014) First mouse model for combined osteogenesis imperfecta and Ehlers-Danlos syndrome. J Bone Miner Res 29:1412–1423
Li H, Jiang X, Delaney J, Franceschetti T, Bilic-Curcic I, Kalinovsky J, Lorenzo JA, Grcevic D, Rowe DW, Kalajzic I (2010) Immature osteoblast lineage cells increase osteoclastogenesis in osteogenesis imperfecta murine. Am J Pathol 176:2405–2413
Gioia R, Panaroni C, Besio R et al (2012) Impaired osteoblastogenesis in a murine model of dominant osteogenesis imperfecta: a new target for osteogenesis imperfecta pharmacological therapy. Stem Cells 30:1465–1476
Coleman RM, Aguilera L, Quinones L, Lukashova L, Poirier C, Boskey A (2012) Comparison of bone tissue properties in mouse models with collagenous and non-collagenous genetic mutations using FTIRI. Bone 51:920–928
Camacho NP, Landis WJ, Boskey AL (1996) Mineral changes in a mouse model of osteogenesis imperfecta detected by Fourier transform infrared microscopy. Connect Tissue Res 35:259–265
Brotto M, Johnson ML (2014) Endocrine crosstalk between muscle and bone. Curr Osteop Rep 12:135–141
Hamrick MW, Shi X, Zhang W, Pennington C, Thakore H, Haque M, Kang B, Isales CM, Fulzele S, Wenger KH (2007) Loss of myostatin (GDF8) function increases osteogenic differentiation of bone marrow-derived mesenchymal stem cells but the osteogenic effect is ablated with unloading. Bone 40:1544–1553
Elkasrawy M, Immel D, Wen X, Liu X, Liang LF, Hamrick MW (2012) Immunolocalization of myostatin (GDF-8) following musculoskeletal injury and the effects of exogenous myostatin on muscle and bone healing. J Histochem Cytochem : Off J Histochem Soc 60:22–30
Hamrick MW, Arounleut P, Kellum E, Cain M, Immel D, Liang LF (2010) Recombinant myostatin (GDF-8) propeptide enhances the repair and regeneration of both muscle and bone in a model of deep penetrant musculoskeletal injury. J Trauma 69:579–583
Verrecchia F, Mauviel A (2004) TGF-beta and TNF-alpha: antagonistic cytokines controlling type I collagen gene expression. Cell Signal 16:873–880
Ghosh AK (2002) Factors involved in the regulation of type I collagen gene expression: implication in fibrosis. Exp Biol Med (Maywood) 227:301–314
Hosaka YZ, Ishibashi M, Wakamatsu J, Uehara M, Nishimura T (2012) Myostatin regulates proliferation and extracellular matrix mRNA expression in NIH3T3 fibroblasts. Biomed Res 33:355–361
Li ZB, Kollias HD, Wagner KR (2008) Myostatin directly regulates skeletal muscle fibrosis. J Biol Chem 283:19371–19378
Elashry MI, Collins-Hooper H, Vaiyapuri S, Patel K (2012) Characterisation of connective tissue from the hypertrophic skeletal muscle of myostatin null mice. J Anat 220:603–611
Mendias CL, Bakhurin KI, Faulkner JA (2008) Tendons of myostatin-deficient mice are small, brittle, and hypocellular. Proc Natl Acad Sci U S A 105:388–393
McBride DJ Jr, Choe V, Shapiro JR, Brodsky B (1997) Altered collagen structure in mouse tail tendon lacking the alpha 2(I) chain. J Mol Biol 270:275–284
Chiu CS, Peekhaus N, Weber H, et al. (2013) Increased muscle force production and bone mineral density in ActRIIB-Fc-treated mature rodents. J Gerontol A Biol Sci Med Sci
Bialek P, Parkington J, Li X et al (2014) A myostatin and activin decoy receptor enhances bone formation in mice. Bone 60:162–171
Acknowledgments
We would like to thank the Biomolecular Imaging Center at the Harry S. Truman Memorial Veterans Hospital and the University of Missouri Comparative Orthopedics Laboratory for their contributions to this project as well as Dr. Mark Ellersieck for his invaluable assistance with statistical analyses. We would also like to thank the following funding sources: National Institutes of Health Grants AR055907 (SMC, BAG, AKO, CER, CLP) and T32RR007004 (BAG), National Space and Biomedical Research Institute Postdoctoral Fellowship NCC 9–58 (SMC), Leda J. Sears Trust Foundation Grant (SMC, BAG, CER, CLP), Phi Zeta Grant (BAG), University of Missouri Life Sciences Fellowship (AKO), University of Missouri Research Board Grant (MB, CLP), and University of Missouri Interdisciplinary Intercampus Research Program (CLP,YW, AKO). The funding sources were not involved in the designing, execution, analysis, reporting, or submission of this work for publication.
Conflicts of interest
Arin Kettle Oestreich, Stephanie Michelle Carleton, Xiaomei Yao, Bettina A. Gentry, Chad Edward Raw, Marybeth Brown, Ferris Michael Pfeiffer, Yong Wang, and Charlotte Longacre Phillips declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oestreich, A.K., Carleton, S.M., Yao, X. et al. Myostatin deficiency partially rescues the bone phenotype of osteogenesis imperfecta model mice. Osteoporos Int 27, 161–170 (2016). https://doi.org/10.1007/s00198-015-3226-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-015-3226-7