Abstract
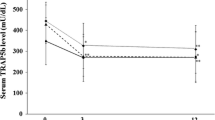
The absorption of bisphosphonates from the gut is poor. The question arises whether the absorption of alendronate, and thus its bioavailability, is further altered by the local inflammatory process in patients with Crohn’s disease, thereby potentially affecting clinical outcome when used in the treatment of osteoporosis. To address this question, urinary excretion of alendronate was evaluated 3 months and 6 months after start of treatment with oral alendronate at a dose of 10 mg/day in 19 osteoporotic patients with stable Crohn’s disease, 12 of whom had an intestinal resection. Biochemical parameters of bone turnover and BMD were also measured at start and at 6 months. Thirteen patients had been previously treated with glucocorticoids and five were currently using them. The average 24-h urinary excretion of alendronate was 0.5–0.6% of the dose administered, a figure comparable to that reported for osteoporotic patients without gut pathology. There was a significant decrease from baseline in urine N-telopeptides of collagen cross-links (NTx)/creatinine (60%) associated with an increase in lumbar spine BMD of already 2% after 6 months of treatment. Our data suggest that in patients with Crohn’s disease, alendronate is adequately absorbed from the intestine and retained in the skeleton. This adequacy is confirmed by appropriate suppression of bone resorption and increase in lumbar spine BMD. These data hold significant implications for the clinical management of patients with Crohn’s disease and osteoporosis.



Similar content being viewed by others
References
Bisschoff SC, Herrmann A, Goeke M, Manns MP, von zur Muehlen A, Brabant G (1997) Altered bone metabolism in inflammatory bowel disease. Am J Gastroenterol 92:1157–1163
Cummings SR, Black DM, Thompson DE, Applegate WB, Barrett-Connor E, Musliner TA et al (1998) Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures—Results from the fracture intervention trial. JAMA 280:2077–2082
Saag KG, Emkey R, Schnitzer TJ, Brown JP, Hawkins F, Goemaere S et al (1998) Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. N Engl J Med 339:292–299
Orwoll E, Ettinger M, Weiss S, Miller P, Kendler D, Graham J et al (2000) Alendronate for the treatment of osteoporosis in men. N Engl J Med 604:604–610
Gertz BJ, Holland SD, Kline WF, Matuszewski BK, Freeman A, Quan H et al (1995) Studies on the oral bioavailability of alendronate. Clin Pharmacol Ther 58:288-298
Mitchell DY, Eusebio RA, Dunlap LE, Pallone KA, Nesbitt JD, Russell DA et al (1998) Risedronate gastrointestinal absorption is independent of site and rate of administration. Pharm Res 15:228–232
Lin JH (1996) Bisphosphonates: A review of their pharmacokinetic properties. Bone 18:75–85
Leder BZ, Kronenberg HM (2000) Gastroenterologists and choosing the right bisphosphonate. Gastroenterology 119:866–869
Haderslev KV, Tjellesen L, Sorensen HA, Staun M (2000) Alendronate increases lumbar spine bone mineral density in patients with Crohn’s disease. Gastroenterology 119:639–646
Sparidans RW, den Hartigh J, Beijnen JH, Vermeij P (1998) Semi-automatic liquid chromatographic analysis of pamidronate in serum and citrate plasma after derivatization with 1-naphthylisothiocyanate. J Chromatogr B Biomed Sci Appl 705:331–339
Sparidans RW, den Hartigh J, Cremers SCLM, Beijnen JH, Vermeij P (1999) Semi-automatic liquid chromatographic analysis of pamidronate in urine after derivatization with 1-naphthylisothiocyanate. J Chromatogr B Biomed Sci Appl 730:95–99
Gubbins PO, Bertch KE (1991) Drug absorption in gastrointestinal disease and surgery. Clin Pharmacokinet 21:431–447
Cremers SCLM, Sparidans RW, den Hartigh J, Hamdy NAT, Vermeij P, Papapoulos SE (2002) A pharmacokinetic and pharmacodynamic model for intravenous bisphosphonate (pamidronate) in osteoporosis. Eur J Clin Pharmacol 57:883–890
McClung M, Clemmesen B, Daifotis A, Gilchrist NL, Eisman J, Weinstein RS et al (1998) Alendronate prevents postmenopausal bone loss in women without osteoporosis—A double-blind, randomized, controlled trial. Ann Intern Med 128:253–261
Garnero P, Darte C, Delmas PD (1999) A model to monitor the efficacy of alendronate treatment in women with osteoporosis using a biochemical marker of bone turnover. Bone 24:603–609
Adachi JD, Saag KG, Delmas PD et al (2001)Two-year effects of alendronate on bone mineral density and vertebral fracture in patients receiving glucocorticoids: a randomized, double-blind, placebo-controlled extension trial. Arthritis Rheum 44:202–11
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cremers, S.C.L.M., van Hogezand, R., Bänffer, D. et al. Absorption of the oral bisphosphonate alendronate in osteoporotic patients with Crohn’s disease. Osteoporos Int 16, 1727–1730 (2005). https://doi.org/10.1007/s00198-005-1911-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-005-1911-7