Abstract
Aims/hypothesis
We have previously shown a negative correlation between serum bilirubin levels and prevalence of type 2 diabetes, suggesting that bilirubin inhibits development of this disease. To confirm this hypothesis, we investigated whether administration of biliverdin, the precursor of bilirubin, protects against the deterioration of glucose tolerance in db/db mice, a rodent model of type 2 diabetes.
Methods
Biliverdin (20 mg/kg daily) was orally administered to 5-week-old db/db mice for 4 weeks. After 4 weeks of treatment, i.p. glucose tolerance and insulin tolerance tests were performed. Insulin content was evaluated by immunostaining and ELISA. Oxidative stress markers (8-hydroxy-2′-deoxyguansosine and dihydroethidium staining) and expression of NADPH oxidase components Pdx1 and Bax were also evaluated in isolated islets.
Results
Treatment with biliverdin partially prevented worsening of hyperglycaemia and glucose intolerance in db/db mice. This effect was accompanied by a significant increase in insulin content and Pdx1 expression, and a significant decrease of apoptosis and Bax expression in pancreatic islets from db/db mice. At the same time, levels of oxidative stress markers and NADPH oxidase component production in islets were normalised. Biliverdin had little effect on HOMA of insulin resistance or insulin resistance evaluated by insulin tolerance tests.
Conclusions/interpretation
Biliverdin may protect against progressive worsening of glucose tolerance in db/db mice, mainly via inhibition of oxidative stress-induced beta cell damage.
Similar content being viewed by others
Introduction
Accumulating evidence shows that oxidative stress is increased in pancreatic beta cells in animal models of and in patients with type 2 diabetes [1–3], implying that oxidative stress contributes to progressive beta cell damage in this condition. It has also been proposed that oxidative stress is implicated in insulin resistance [4, 5]. In fact, several antioxidants have been reported to improve beta cell damage and insulin sensitivity in animal models of type 2 diabetes [4, 6, 7]. Taken together, these findings indicate that oxidative stress is closely associated with the development of type 2 diabetes. However, the causative role of oxidative stress remains controversial because there is little evidence from humans showing the beneficial effects of antioxidants on development of type 2 diabetes.
Bilirubin is an end-product of haem catabolism and has long been used in clinical practice as a marker of hepatobiliary and haematopoietic diseases. Recently, much interest has been drawn to its antioxidative effect, particularly in relation to atherosclerotic diseases and diabetic vascular complications. Indeed, a number of studies have shown that higher concentrations of serum bilirubin are associated with decreased risk of coronary artery disease and stroke [8–11]. We previously reported that the prevalence of vascular complications, including retinopathy, nephropathy and coronary artery disease, was markedly lower in diabetic patients with Gilbert syndrome, a congenital hyperbilirubinaemia [12]. The antioxidative effect of bilirubin was first recognised in an in vitro experiment showing it to be a scavenger of peroxyl radicals and in which its activity was equal to or more potent than that of α-tocopherol [13]. We therefore speculated that bilirubin might protect against the development of type 2 diabetes. In this context, we recently found that serum bilirubin concentrations were inversely associated with HbA1c levels and high-sensitivity C-reactive protein levels, and that higher concentrations of serum bilirubin were associated with decreased prevalence of type 2 diabetes in a large cross-sectional study of middle-aged and elderly Japanese men and women (n = 12,400) [14]. Similar findings were also reported in a large cross-sectional study in a Korean population (n = 93,909) [15]. These findings suggest that higher concentrations of bilirubin might confer protection against development of type 2 diabetes. However, these association studies were not able to confirm a causative role of bilirubin in the development of type 2 diabetes. Therefore, in this study, we investigated whether administration of biliverdin, the precursor of bilirubin, protects against the progressive deterioration of glucose tolerance in db/db mice, a rodent model of type 2 diabetes. We also explored the underlying mechanisms.
Methods
Animals
Male C57BL/KsJ db/db mice and age-matched lean littermate db/+ mice were purchased from Clea (Tokyo, Japan). All mice were bred under pathogen-free conditions at Kyusyu University Animal Center, Fukuoka, Japan. The animals had free access to tap water and standard chow (Clea). At 5 weeks of age, half of the db/db (n = 8) and half of the db/+ (n = 8) mice were randomly chosen to receive a powdered diet (Clea) supplemented with biliverdin (20 mg/kg) (Frontier Scientific, Logan, UT, USA) for 4 weeks, while the remaining mice received a powdered diet without biliverdin over the same time period. The diet was stored at 4°C and kept away from excessive light. All protocols were reviewed and approved by the Committee on the Ethics of Animal Experiments, Graduate School of Medical Science, Kyushu University.
Measurement of blood glucose, and i.p. glucose tolerance and insulin tolerance tests
Blood samples were obtained from the mouse tail vein. Plasma glucose concentrations were determined by the glucose oxidase method and plasma insulin concentrations by ELISA (Morinaga Institute of Biological Science, Yokohama, Japan). After a 16 h fast, glucose tolerance was assessed by an i.p. glucose tolerance test (IPGTT). Briefly, under anaesthesia with 2% (vol./vol.) isoflurane via facemask for 1.5 to 2 min, a glucose bolus (0.5 g/kg i.p.) was injected and blood samples collected from the tail vein at 0, 15, 30, 60, 90 and 120 min. This method of anaesthesia allows mice to completely recover between tail cuts and has minimal effects on glucose levels. For the insulin tolerance test (ITT), the mice were injected with 2 units/kg of human biosynthetic insulin (Novo Nordisk, Bagsvaerd, Denmark) and blood samples were collected at 0, 30, 60, 90, 120 and 150 min, as described above. HOMA of insulin resistance (HOMA-IR) was calculated as described by Matthews et al. [16]
Immunohistochemistry
Immunostaining for 8-hydroxy-2′-deoxyguanosine (8-OHdG), a marker of oxidative DNA damage, and gp91phox, a major component of NADPH oxidase, was carried out as previously described [17, 18]. Briefly, the pancreas was fixed in 10% (vol./vol.) formaldehyde and embedded in paraffin. Paraffin sections (3 μm) were cut and deparaffinised. After inactivation of endogenous peroxidase with 3% (vol./vol.) H2O2 in methanol for 15 min at room temperature, the sections were preincubated for 30 min with 1% (wt/vol.) BSA in PBS. Samples were incubated overnight at 4°C with anti-human 8-OHdG goat polyclonal antibodies (0.2 μg/ml) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) or anti-human p91phox goat polyclonal antibodies (0.2 μg/ml), washed in PBS and probed with anti-goat peroxidase-labelled IgG antibodies (Histofine Simple Stain MAX PO[G]; Nichirei, Tokyo, Japan) for 30 min at room temperature. The primary antibodies were replaced with normal goat serum as a negative control. Sections were counterstained with haematoxylin and examined under a light microscope. Peroxidase abundance was then visualised with diaminobenzidine. For insulin staining, the samples were incubated with anti-human insulin guinea pig polyclonal antibody (100 μg/ml) (Wako Pure Chemical, Osaka, Japan).
Immunostaining images of five islets contained in five consecutive cross-sections of the pancreas of each mouse were used for quantitative evaluation. Images were saved as PICT images, converted to grey-scale images using Adobe Photoshop (Adobe Systems, San Jose, CA, USA) and staining intensities were quantitatively analysed using NIH image software (http://rsbweb.nih.gov/ij/download.html).
Dihydroethidium staining
To detect superoxide production, pancreas tissue was stained with dihydroethidium (Invitrogen, Carlsbad, CA, USA), as previously described [19]. Briefly, mice were intravenously administered 1 ml dihydroethidium (1 mg/ml in PBS) through the right jugular vein under isoflurane anaesthesia. At 2 h after injection, the mice were killed by transcardial perfusion with 50 ml 4% (vol./vol.) formaldehyde in PBS. The pancreas was frozen immediately in OCT compound (Tissue-Tech II; Sakura Fine Chemical, Tokyo, Japan) and sectioned (10 μm thick) on a cryostat. Nuclear staining was then performed using Hoechst 33258 (Invitrogen) in PBS for 15 min in a dark chamber and sections were mounted after rinsing in distilled H2O. Fluorescence images were obtained using a fluorescence microscope (BZ-9000; Keyence, Osaka, Japan). The relative fluorescence intensity in islets was quantified using Adobe Photoshop (Adobe Systems). We manually selected islets in all ×200 images and recorded the mean value of the histogram on the red colour channel as the fluorescent level of dihydroethidium in each islet. The average islet fluorescence level in each image was compared between groups.
Pancreatic islet isolation
Islets were isolated from the pancreas as previously described [20]. Briefly, diabetic and control mice at 9 weeks of age were killed and the pancreas was immediately dissected, placed in cold Hanks’ solution (Sigma Aldrich, St Louis, MO, USA) and cut into small pieces. These pieces were transferred to vials containing collagenase solution (Roche Molecular Biochemicals, Mannheim, Germany). The digest was then washed three times by filling the vial with Hanks’ solution. The islets were then selected under a light microscope. Insulin content in the islets was measured by ELISA as described above and adjusted by DNA content.
Detection of superoxide using dihydroethidium in pancreatic islets ex vivo
Isolated pancreatic islets from male db/db mice (Clea) were incubated at 37°C for 24 h in DMEM containing 10% (vol./vol.) FBS and 25 mmol/l glucose, with or without 1 × 10−6 mol/l biliverdin. The islets were then loaded with 20 μmol/l dihydroethidium. After 30 min, fluorescence images were obtained using a fluorescence microscope (Keyence). Fluorescence intensities were quantitatively analysed using Adobe Photoshop (Adobe Systems).
RNA extraction and quantitative RT-PCR for gene expression in islets
Gene expression of insulin, Pdx1, calcyclin, synaptosomal-associated protein of 25 kDa (Snap25), Bax, activating transcription factor 3 (Atf3), Fas, glutathione peroxidase-1 (Gpx [also known as Gpx1]), gp91phox (also known as Cybb) and NA(D)PH oxidase components in islet extract was determined by real-time quantitative RT-PCR performed on a Chromo4 real-time PCR detector (Bio-Rad Laboratories, Hercules, CA, USA) with iQ SYBR Green Supermix reagent (Bio-Rad). Total RNA was extracted from frozen pancreatic islet samples using Isogen (Nippon Gene, Tokyo, Japan). Extracted RNA (4 μg) was converted to single-stranded cDNA using Superscript III reverse transcriptase (Invitrogen). PCR reactions for each target cDNA were done at the conditions shown in Electronic supplementary material (ESM) Table 1 using specific primers. β-Actin was used as internal control. The specificity of PCR amplification was confirmed by melting curve analysis and agarose gel electrophoresis.
Apoptosis detection
Islet cell apoptosis was determined by TUNEL staining using an in situ detection kit (Wako). Pancreatic sections were also immunostained for insulin, as described above. The results are expressed as the percentage of TUNEL-positive beta cells.
Statistical analysis
Data are expressed as means ± SE. Differences between groups were analysed using two-tailed Student’s t test. Multiple comparisons between groups were conducted by one-way ANOVA with Fisher’s probable least-squares difference test for post hoc analysis. Values of p < 0.05 were considered significant.
Results
Body weight and fasting glucose levels
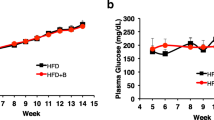
Biliverdin (20 mg/kg daily) was orally administered to 5-week-old db/db mice for 4 weeks. As shown in Fig. 1a, body weight and food intake (ESM Table 2) in db/db mice were significantly greater at 0 and at 4 weeks of treatment than in age-matched db/+ mice and were not significantly affected by biliverdin treatment. Fasting glucose levels were significantly lower in biliverdin-treated than in untreated db/db mice after 4 weeks of treatment (Fig. 1b). There was no significant difference in serum levels of bilirubin between the three groups at 0 and 4 weeks (ESM Table 2).
Changes in body weight (a) and fasting glucose (b) after 4 weeks of treatment in db/db mice (white circles), biliverdin-treated db/db mice (black triangles), db/+ mice (black circles) and biliverdin-treated db/+ mice (white triangles). Results are expressed as means ± SE; n = 8 mice/group, *p < 0.05
IPGTT, ITT and HOMA of insulin resistance
IPGTTs were performed after 4 weeks of treatment. After a bolus injection of glucose (0.5 mg/kg i.p.), the blood glucose level was significantly lower at 30, 60, 90 and 120 min (Fig. 2a) and the AUC was significantly lower in biliverdin-treated than in untreated db/db mice (ESM Fig. 1a). In contrast, plasma insulin levels were significantly higher at 90 and 120 min (Fig. 2b) in biliverdin-treated than in untreated db/db mice, as was the AUC (ESM Fig. 1b).
Effects of biliverdin on blood glucose and insulin resistance. a Blood glucose levels after an IPGTT in db/db mice (white circles), biliverdin-treated db/db mice (black triangles), db/+ mice (black circles) and biliverdin-treated db/+ mice (white triangles). b Plasma insulin levels after an IPGTT in mice as above (a). c Changes in blood glucose levels after an ITT in mice as above (a), except biliverdin-treated db/+ mice. Blood glucose levels are expressed as percentage of basal blood glucose levels. Results (a–c) are expressed as means ± SE; n = 8 mice/group; *p < 0.05, **p < 0.01
In the ITT, insulin sensitivity was markedly worse in db/db mice than in control db/+ mice. Biliverdin treatment slightly, but significantly improved the insulin-induced reduction of blood glucose levels at 60 min (Fig. 2c). HOMA-IR was markedly increased in db/db mice and was slightly, but not significantly reduced by biliverdin treatment (ESM Fig. 1c).
Insulin content in pancreatic islets
Insulin content in pancreatic sections was evaluated by immunohistochemistry and in isolated islets by ELISA. The immunostaining intensities of insulin were decreased in islets from db/db mice compared with db/+ mice. This decrease was significantly reversed by biliverdin treatment (Fig. 3, ESM Fig. 2a). Similarly, insulin content measured by ELISA was significantly decreased in isolated islets from db/db mice and was completely reversed by biliverdin treatment (ESM Fig. 2b). mRNA expression of Pdx1 (a critical regulator of insulin promoter activity), calcyclin (a Ca2+-binding protein), Snap25 (a t-SNARE protein) and Bax (a regulator of apoptosis) was evaluated in isolated islets. Expression of transcription factors or genes implicated in distal secretory mechanisms was not altered or even increased in db/db mice, but was further enhanced by biliverdin treatment (Fig. 4a–c). Bax mRNA was significantly increased in db/db mice. This increase was completely restored by biliverdin treatment (Fig. 4d). Expression changes of other apoptosis-related (Atf3, Fas) and oxidative stress-related genes (Gpx) were also reversed by biliverdin treatment (Fig. 4e–g). Pancreatic beta cell apoptosis was evaluated by TUNEL assay. Approximately one to three TUNEL-positive cells were observed in islets from db/db mice, while very few TUNEL-positive cells were found in islets from control mice or biliverdin-treated db/db mice (Fig. 4h).
Representative photomicrographs of pancreatic islet sections immunostained with anti-insulin antibody after 4 weeks of biliverdin treatment. Scale bar 100 μm. Immunostaining images of five islets contained in five consecutive cross-sections in the pancreas of each mouse were used for quantitative evaluation. Results are expressed as mean ± SE percentages of the levels in control db/+ mice; n = 8 mice/group
a mRNA expression levels of (a) Pdx1, (b) calcyclin, (c) Snap25, (d) Bax, (e) Atf3, (f) Fas and (g) Gpx in isolated islets. Total RNA was extracted from islets isolated from db/+, db/db and biliverdin-treated db/db mice after 4 weeks of treatment. mRNA levels were measured by real-time RT-PCR and adjusted for the levels of β-actin. Results are expressed as mean ± SE percentages of the levels in control db/+ mice; n = 8 mice/group; *p < 0.05, **p < 0.01 and ***p < 0.001. h Representative photomicrographs of apoptotic cells in islets from mice as labelled, assessed by TUNEL assay after 4 weeks of treatment. Apoptotic cells are indicated by red arrows
Oxidative stress in islets
Oxidative stress in islets was evaluated by immunostaining with 8-OHdG. The immunostaining intensity was significantly increased in db/db mice, this increase being completely normalised by biliverdin treatment (Fig. 5a, b). These findings were also confirmed by dihydroethidium staining, a relatively specific marker of superoxide production. The oxidised dihydroethidium signals were much higher in db/db mice than in control mice. Biliverdin treatment appeared to completely normalise oxidised dihydroethidium signals in the islets of db/db mice to the control levels (Fig. 5c). In an ex vivo experiment using isolated islets, biliverdin treatment (1 × 10−6 mol/l) also significantly inhibited the oxidised dihydroethidium signals induced by 25 mmol/l glucose levels (Fig. 5d, e).
a, b Effect of biliverdin on 8-OHdG accumulation. Representative photomicrographs (a) and immunostaining intensity (b) of pancreatic islet sections from animals as labelled, immunostained with anti-8-OHdG antibody after 4 weeks of biliverdin treatment. Scale bar (a) 100 μm. Immunostaining images of five islets contained in five consecutive cross-sections in the pancreas of each mouse were used for quantitative evaluation (b). Results (b) are expressed as mean ± SE percentages of the levels in control db/+ mice; *p < 0.05 and **p < 0.01 vs db/db mice. c Detection of superoxide using dihydroethidium staining in situ. Images are representative photomicrographs of pancreatic islet sections stained with dihydroethidium. Scale bar, 100 μm. Red, dihydroethidium fluorescence; blue, Hoechst 33258 (nuclear). d Effect of biliverdin treatment on superoxide production evaluated by dihydroethidium staining in pancreatic islets ex vivo. Scale bar, 100 μm. Representative photomicrographs are shown, with (e) relative fluorescence intensity of isolated pancreatic islets. Data (e) are expressed as mean percentage of control ± SE; n = 15; ***p < 0.001
NAD(P)H oxidase gp91phox has been reported to be produced in pancreatic beta cells and is a major source of superoxide production in islets. In this study, we evaluated the abundance of gp91phox. The immunostaining intensity of gp91phox was significantly increased in islets from db/db mice, an increase completely normalised by biliverdin treatment (Fig. 6). In parallel, gp91phox mRNA levels were significantly increased in isolated islets from db/db mice. Here, too, this increase was normalised by biliverdin treatment (ESM Fig. 3a). Similar changes were observed for mRNA expression of other NAD(P)H oxidase component genes (Nox4, p47phox [also known as Ncf1], p22phox [also known as Cyba]) (ESM Fig. 3b–d).
a Representative photomicrographs and (b) relative immunostaining intensity of pancreatic islet sections as labelled, immunostained with anti-gp91phox antibody after 4 weeks of biliverdin treatment. Scale bar, 100 μm. Immunostaining images of five islets contained in five consecutive cross-sections in the pancreas of each mouse were used for quantitative evaluation (b). Results are expressed as mean ± SE percentages of the levels in control db/+ mice; n = 8 mice/group; ***p < 0.001 vs db/db mice
Discussion
There is increasing evidence that oxidative stress is closely associated with the development of type 2 diabetes [1–3]. However, there is very little evidence showing the beneficial effects in humans of antioxidants on the development of type 2 diabetes. We and others have found that higher concentrations of serum bilirubin are associated with decreased prevalence of type 2 diabetes in large cross-sectional studies [14, 15], suggesting that bilirubin is an effective antioxidant that can protect against the development of type 2 diabetes. In the present study, we investigated this hypothesis and found that administration of biliverdin to 5-week-old db/db mice partially prevented the progressive deterioration of glucose tolerance. Genetically, db/db mice lack the leptin receptor and exhibit severe leptin resistance. This model resembles human type 2 diabetes with peripheral insulin resistance and progressive pancreatic beta cell deterioration. In fact, both HOMA-IR and ITT demonstrated marked insulin resistance in db/db mice. Reactive oxygen species (ROS) have been reported to activate multiple serine kinase cascades. For example, an increase in serine phosphorylation of the insulin receptor substrate proteins IRS-1 and IRS-2 by ROS decreased the extent of tyrosine phosphorylation, thereby attenuating insulin action [21]. In this study, biliverdin treatment improved insulin sensitivity in db/db mice, albeit by a small magnitude. We also measured serum adiponectin levels, but the decreased levels of serum adiponectin in db/db mice were not significantly affected by biliverdin treatment (data not shown). On the other hand, biliverdin significantly increased the insulin content in islets from db/db mice to near control levels. Simultaneously, biliverdin induced a significant increase in Pdx1 expression and a significant decrease in Bax expression in islets from db/db mice. Whereas chronic oxidative stress has been reported to decrease insulin gene expression via downregulation of PDX1 and to accelerate beta cell apoptosis [1], the present findings suggest that the beneficial effects of biliverdin on glucose intolerance in db/db mice may be due to the inhibition of oxidative stress and the subsequent increase of insulin synthesis by upregulation of Pdx1 expression and/or the suppression of beta cell apoptosis. This is consistent with a previous finding that the reduction of beta cell oxidative stress induced by overexpression of glutathione peroxidase-1 is protective in db/db mice [22]. However, various insulin secretory genes are also known to be altered in db/db mice [23]. In fact, this study showed that biliverdin treatment affected expression of exocytosis genes as well as transcription genes, suggesting that biliverdin treatment might restore such secretory dysfunction in db/db mice (Fig. 4). Further studies should be done to clarify these mechanisms in detail.
In the present study, mice were orally treated with biliverdin rather than bilirubin, because biliverdin is more readily water-soluble than bilirubin. Serum biliverdin can quickly enter cells and is converted to bilirubin by biliverdin reductase [24]. Therefore, it is likely that the beneficial effects of biliverdin are mediated by increased intracellular bilirubin levels generated from exogenously administered biliverdin, rather than from increased serum biliverdin levels. We recently showed that biliverdin administration for 8 weeks starting at 12 weeks of age normalised renal function and histological abnormalities in kidneys from db/db mice by reducing oxidative stress in renal tissues [19]. In that study, there was no significant increase in serum bilirubin levels. Increased mitochondrial superoxide production has been suggested to be the main source of ROS in diabetic beta cells [25]. The present study suggests that increased intracellular bilirubin levels may inhibit ROS production via its strong radical scavenging activity. In diabetic vasculature tissues, we and other investigators have suggested that NAD(P)H oxidase may be an important source of increased oxidative stress [26–28]. Several studies have demonstrated the presence of the NAD(P)H oxidase components gp91phox and NAD(P)H oxidase 4, and of other cytosolic regulatory components in rat pancreatic islets [29]. Other emerging evidence shows that inappropriate activation of NAD(P)H oxidase may damage pancreatic beta cells, leading to progressive deterioration of glucose tolerance in type 2 diabetes [30, 31]. We previously reported high glucose levels and glibenclamide-stimulated ROS production in the pancreatic beta cell line MIN6, via protein kinase C-dependent activation of NAD(P)H oxidase [32]. Increased levels of NAD(P)H oxidase have also been shown in several animal models of type 2 diabetes [18]. Collectively, these results suggest that NAD(P)H oxidase plays critical roles in increased oxidative stress in diabetic islets. However, NAD(P)H oxidase is not the sole source of oxidative stress in db/db mice islets, and alterations of antioxidant gene expression, e.g. Gpx, may also play an important role. Therefore, normalisation of NAD(P)H oxidase by biliverdin treatment may at least in part account for the strong antioxidative effect and protective effect of biliverdin on the deterioration of glucose tolerance.
In conclusion, we have shown for the first time that biliverdin administration partially protects against the deterioration of glucose tolerance in db/db mice, mainly via inhibition of oxidative stress and subsequent protection of pancreatic beta cells. Taken together with evidence from humans showing a negative correlation between serum bilirubin levels and the prevalence of type 2 diabetes, the present findings reveal a potential inhibitory effect of bilirubin on the development of type 2 diabetes. Thus, serum bilirubin levels may be used as a predictor of development of type 2 diabetes. More interestingly, compounds with similar properties to bilirubin may lead to a new class of therapeutic agents capable of protecting against the development of type 2 diabetes.
Abbreviations
- HOMA-IR:
-
HOMA of insulin resistance
- IPGTT:
-
i.p. glucose tolerance test
- ITT:
-
Insulin tolerance test
- 8-OHdG:
-
8-Hydroxy-2′-deoxyguanosine
- ROS:
-
Reactive oxygen species
References
Robertson RP (2004) Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet beta cells in diabetes. J Biol Chem 279:42351–42354
Ihara Y, Toyokuni S, Uchida K et al (1999) Hyperglycemia causes oxidative stress in pancreatic beta-cells of GK rats, a model of type 2 diabetes. Diabetes 48:927–932
Sakuraba H, Mizukami H, Yagihashi N, Wada R, Hanyu C, Yagihashi S (2002) Reduced beta-cell mass and expression of oxidative stress-related DNA damage in the islet of Japanese type II diabetic patients. Diabetologia 45:85–96
Houstis N, Rosen ED, Lander ES (2006) Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 440:944–948
Paolisso G, Giugliano D (1996) Oxidative stress and insulin action: is there a relationship? Diabetologia 39:357–363
Evans JL, Goldfine ID, Maddux BA, Grodsky GM (2003) Are oxidative stress-activated signaling pathways mediators of insulin resistance and beta-cell dysfunction? Diabetes 52:1–8
Rudich A, Tirosh A, Potashnik R, Khamaisi M, Bashan N (1999) Lipoic acid protects against oxidative stress induced impairment in insulin stimulation of protein kinase B and glucose transport in 3T3-L1 adipocytes. Diabetologia 42:949–957
Schwertner HA, Jackson WG, Tolan G (1994) Association of low serum concentration of bilirubin with increased risk of coronary artery disease. Clin Chem 40:18–23
Breimer LH, Wannamethee G, Ebrahim S, Shaper AG (1995) Serum bilirubin and risk of ischemic heart disease in middle-aged British men. Clin Chem 41:1504–1508
Hopkins PN, Wu LL, Hunt SC, James BC, Vincent GM, Williams RR (1996) Higher serum bilirubin is associated with decreased risk for early familial coronary artery disease. Arterioscler Thromb Vasc Biol 16:250–255
Perlstein TS, Pande RL, Creager MA, Weuve J, Beckman JA (2008) Serum total bilirubin level, prevalent stroke, and stroke outcomes: NHANES 1999–2004. Am J Med 121:781–788.e1
Inoguchi T, Sasaki S, Kobayashi K, Takayanagi R, Yamada T (2007) Relationship between Gilbert syndrome and prevalence of vascular complications in patients with diabetes. JAMA 298:1398–1400
Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN (1987) Bilirubin is an antioxidant of possible physiological importance. Science 235:1043–1046
Ohnaka K, Kono S, Inoguchi T et al (2010) Inverse associations of serum bilirubin with high sensitivity C-reactive protein, glycated hemoglobin, and prevalence of type 2 diabetes in middle-aged and elderly Japanese men and women. Diabetes Res Clin Pract 88:103–110
Han SS, Na KY, Chae DW, Kim YS, Kim S, Chin HJ (2010) High serum bilirubin is associated with the reduced risk of diabetes mellitus and diabetic nephropathy. Tohoku J Exp Med 221:133–140
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419
Etoh T, Inoguchi T, Kakimoto M et al (2003) Increased expression of NAD(P)H oxidase subunits, NOX4 and p22phox, in the kidney of streptozotocin-induced diabetic rats and its reversibity by interventive insulin treatment. Diabetologia 46:1428–1437
Nakayama M, Inoguchi T, Sonta T et al (2005) Increased expression of NAD(P)H oxidase in islets of animal models of type 2 diabetes and its improvement by an AT1 receptor antagonist. Biochem Biophys Res Commun 332:927–933
Fujii M, Inoguchi T, Sasaki S et al (2010) Bilirubin and biliverdin protect rodents against diabetic nephropathy by downregulating NAD(P)H oxidase. Kidney Int 78:905–919
Andersson A (1978) Isolated mouse pancreatic islets in culture: effects of serum and different culture media on the insulin production of the islets. Diabetologia 14:397–404
Paz K, Hemi R, LeRoith D et al (1997) A molecular basis for insulin resistance. Elevated serine/threonine phosphorylation of IRS-1 and IRS-2 inhibits their binding to the juxtamembrane region of the insulin receptor and impairs their ability to undergo insulin-induced tyrosine phosphorylation. J Biol Chem 272:29911–29918
Harmon JS, Bogdani M, Parazzoli SD et al (2009) β-Cell-specific overexpression of glutathione peroxidase preserves intranuclear MafA and reverses diabetes in db/db mice. Endocrinology 150:4855–4862
Kjorholt C, Akerfeldt MC, Biden TJ, Laybutt DR (2005) Chronic hyperglycemia, independent of plasma lipid levels, is sufficient for the loss of beta-cell differentiation and secretory function in the db/db mouse model of diabetes. Diabetes 54:2755–2763
Stocker R (2004) Antioxidant activities of bile pigments. Antioxid Redox Signal 6:841–849
Green K, Brand MD, Murphy MP (2004) Prevention of mitochondrial oxidative damage as a therapeutic strategy in diabetes. Diabetes 53(Suppl 1):S110–S118
Inoguchi T, Li P, Umeda F et al (2000) High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C-dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes 49:1939–1945
Kim YK, Lee MS, Son SM et al (2002) Vascular NADH oxidase is involved in impaired endothelium-dependent vasodilation in OLETF rats, a model of type 2 diabetes. Diabetes 51:522–527
Inoguchi T, Sonta T, Tsubouchi H et al (2003) Protein kinase C-dependent increase in reactive oxygen species (ROS) production in vascular tissues of diabetes: role of vascular NAD(P)H oxidase. J Am Soc Nephrol 14:S227–S232
Oliveira HR, Verlengia R, Carvalho CR, Britto LR, Curi R, Carpinelli AR (2003) Pancreatic beta-cells express phagocyte-like NAD(P)H oxidase. Diabetes 52:1457–1463
Guichard C, Moreau R, Pessayre D, Epperson TK, Krause KH (2008) NOX family NADPH oxidases in liver and in pancreatic islets: a role in the metabolic syndrome and diabetes? Biochem Soc Trans 36:920–929
Newsholme P, Morgan D, Rebelato E et al (2009) Insights into the critical role of NADPH oxidase(s) in the normal and dysregulated pancreatic beta cell. Diabetologia 52:2489–2498
Tsubouchi H, Inoguchi T, Inuo M et al (2005) Sulfonylurea as well as elevated glucose levels stimulate reactive oxygen species production in the pancreatic beta-cell line, MIN6—a role of NAD(P)H oxidase in beta-cells. Biochem Biophys Res Commun 326:60–65
Acknowledgements
This work was supported in part by Special Coordination Funds for Promoting Science and Technology (SCF) (funding programme of the Innovation Center for Medical Redox Navigation), Japan.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Table 1
Nucleotide sequences of primers (PDF 177 kb)
ESM Table 2
Effect of biliverdin on serum bilirubin levels and food intake (PDF 70 kb)
ESM Fig. 1
Effects of biliverdin on blood glucose and insulin resistance. a AUC glucose after an IPGTT in db/+ mice, db/db mice, biliverdin-treated db/+ mice and biliverdin-treated db/db mice. b AUC plasma insulin concentration after an IPGTT in mice as above (a). c HOMA-IR levels after 4 weeks of treatment in mice as above (a), except biliverdin-treated db/+ mice. Results (a–c) are expressed as means ± SE; n = 8 mice/group; **p < 0.01 and ***p < 0.001 (PDF 5556 kb)
ESM Fig. 2
a Relative immunostaining intensity of pancreatic islet sections immunostained with anti-insulin antibody after 4 weeks of biliverdin treatment. Immunostaining images of five islets contained in five consecutive cross-sections in the pancreas of each mouse were used for quantitative evaluation. b Insulin content evaluated by ELISA in islets isolated from mice as labelled. Insulin content was adjusted for DNA content. Results (a, b) are expressed as mean ± SE percentages of the levels in (a) control db/+ mice and (b) untreated db/+ mice; n = 8 mice/group; *p < 0.05, **p < 0.01 and ***p < 0.001 (PDF 11847 kb)
ESM Fig. 3
mRNA expression of (a) gp91phox, (b) Nox4, (c) p47phox and (d) p22phox in islets. Total RNA was extracted from the islets after 4 weeks of biliverdin (BVD) treatment. mRNA levels were measured by real-time RT-PCR and adjusted for levels of β-actin. Results are expressed as mean ± SE percentages of the levels in untreated db/+ mice; n = 8 mice/group; *p < 0.05, **p < 0.01 and ***p < 0.001 (PDF 11847 kb)
Rights and permissions
About this article
Cite this article
Ikeda, N., Inoguchi, T., Sonoda, N. et al. Biliverdin protects against the deterioration of glucose tolerance in db/db mice. Diabetologia 54, 2183–2191 (2011). https://doi.org/10.1007/s00125-011-2197-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-011-2197-2