Abstract
Aims/hypothesis
The LEW.1AR1-iddm rat is an animal model of spontaneous type 1 diabetes mellitus. This study analysed how adoptive transfer of selective T cell subpopulations affects the incidence of diabetes.
Methods
CD4+ or CD8+ T cells were isolated from diabetic LEW.1AR1-iddm rats or diabetes-resistant LEW.1AR1 rats. Cells were selectively transferred into athymic LEW.1AR1-Whn rnu or prediabetic LEW.1AR1-iddm rats. The animals were monitored for blood glucose, islet infiltration and immune cell composition of pancreas-draining lymph nodes.
Results
After adoptive transfer of CD4+ T cells from diabetic LEW.1AR1-iddm rats into athymic LEW.1AR1-Whn rnu rats, 50% of the recipients developed diabetes. Transfer of CD8+ T cells failed to induce diabetes. Only 10% of the athymic recipients became diabetic after co-transfer of CD4+ and CD8+ T cells. Adoptive transfer of CD8+ T cells from LEW.1AR1 or diabetic LEW.1AR1-iddm rats into prediabetic LEW.1AR1-iddm rats significantly reduced the incidence of diabetes. In protected normoglycaemic animals regulatory CD8+/CD25+ and CD4+/CD25+ T cell subpopulations that were also FOXP3-positive accumulated in the pancreas-draining lymph nodes. In this lymphatic organ, gene expression of anti-inflammatory cytokines was significantly higher than in diabetic rats.
Conclusions/interpretation
Our results show that adoptive transfer of CD4+ but not CD8+ T cells from diabetic LEW.1AR1-iddm rats induced diabetes development. Importantly, CD8+ T cells from diabetic LEW.1AR1-iddm rats and diabetes-resistant LEW.1AR1 rats provided protection against beta cell destruction. The accumulation of regulatory T cells in the pancreas-draining lymph nodes from protected rats indicates that transferred CD8+ T cells may have beneficial effects in the control of beta cell autoimmunity.
Similar content being viewed by others
Introduction
The LEW.1AR1-iddm (IDDM) rat is an animal model of autoimmune diabetes [1] with characteristics closely resembling human type 1 diabetes [2]. In contrast to other established rodent models such as the biobreeding diabetes-prone (BB–DP) rat and the NOD mouse, it develops the diabetic syndrome without lymphopenia or without an immune defect in the natural killer (NK)/T-lymphocyte population [1].
The LEW.1AR1-iddm rat originated from the congenic strain LEW.1AR1. Both strains express the recombinant MHC haplotype RT1 r2: RT1-A a B/D u C u . RT1 class II(u) alleles have been demonstrated to confer susceptibility to experimentally induced type 1 diabetes in concert with autoreactive cells and triggering events [3]. The only difference between the LEW.1AR1-iddm rat and the background strain is a mutation on chromosome 1 within Iddm8 (RNO1q43-51) [4, 5]. Adoptive transfer of T cells from diabetic LEW.1AR1-iddm rats to prediabetic LEW.1AR1-iddm rats significantly increased the incidence of diabetes [6] in agreement with studies on NOD mice and BB–DP rats [7–12]. The autoreactive potential can be transferred through immune cells from diabetic LEW.1AR1-iddm rats into athymic nude LEW.1AR1-Whn rnu animals, but not to the diabetes-resistant LEW.1AR1 background strain [6]. On the other hand, it was possible to confer protection against diabetes through adoptive transfer of immune cells from the LEW.1AR1 background strain into prediabetic LEW.1AR1-iddm rats [6]. Thus, regulatory elements of the cellular immune system apparently protect LEW.1AR1 rats against beta cell autoreactive immune cells. However, the specific roles of different T cell subpopulations in this (im)balance between auto-aggression and protection are so far unknown. To address this issue we performed adoptive transfer experiments using purified CD4+ and CD8+ T cell subpopulations from diabetic LEW.1AR1-iddm rats and diabetes-resistant LEW.1AR1 rats to elucidate the potential of these T cells in the development of diabetes.
Our data provide evidence that T cells from the CD8+ subpopulation confer protection after transfer into prediabetic rats, apparently through shifting the T cell balance towards a regulatory phenotype in pancreas-draining lymph nodes.
Methods
Animals
Diabetes-prone LEW.1AR1-iddm rats, diabetes-resistant LEW.1AR1 rats and athymic nude LEW.1AR1-Whn rnu rats were housed in the Central Animal Facility of Hannover Medical School, Hannover, Germany. All animals were maintained and regularly monitored for murine pathogens to document the health status as described before, according to Federation of European Laboratory Animal Science Associations guidelines [6]. Breeding conditions for the LEW.1AR1-iddm colony and the athymic nude LEW.1AR1-Whn rnu rats used for the transfer experiments were as described [6]. The experiments involving animals were approved by the local ethics committee on animal welfare and the study was conducted in accordance with the Principles of Laboratory Care.
Adoptive transfer of T cells
Lymph nodes (cervical and mesenteric) and spleen from donor rats were removed, minced, processed into single-cell suspensions and separated for T cell subpopulation analyses by magnetic cell separation technique [13].
Briefly, CD8+ T cells were labelled with unconjugated CD8-monoclonal antibody (mAb; clone RPA-T8, mouse IgG1) for 30 min on ice. After two washing steps, the anti-CD8 mAb-labelled T cells were incubated for 15 min at 4°C with rat anti-mouse IgG1 paramagnetic microbeads (Miltenyi Biotech, Bergisch Gladbach, Germany). Thereafter, bound CD8+ cells were separated on MS columns (Miltenyi Biotech) using MACS technology according to the protocol of the supplier (Miltenyi Biotech). For isolation of CD4+ T cells, cells were directly incubated for 15 min at 4°C with anti-rat CD4 microbeads (Miltenyi Biotech). After two washing steps, cells were separated using MS depletion columns as described for CD8+ T cells. The cell suspensions were cultured with 10 µg/ml concanavalin A (Biochrom, Berlin, Germany) for 72 h after isolation of specific T cell subpopulations as described before [6] to obtain a sufficient number of T cells for transfer to non-irradiated animals. We used concanavalin A stimulation in accordance with adoptive transfer protocols in the BB rat [11] because until now it has not been possible to identify T cell activating islet autoantigens in this model. The purity of each T cell population was determined by flow cytometry before and after stimulation with concanavalin A and was consistently >98%. The following antibodies were used for flow cytometry: CD4 (OX-38) FITC, CD3 (G4.18) PE, (BD, Heidelberg, Germany); CD8 (OX-8) FITC, CD8 (OX-8) PE, TCR (R73) FITC, CD45RA (OX-33) FITC, NKR (10/78) FITC, ED1 FITC (all from Serotec, Düsseldorf, Germany). To determine the regulatory and auto-aggressive potential, two different adoptive transfer protocols were used: (1) cells were transferred from diabetic LEW.1AR1-iddm donors into athymic nude LEW.1AR1-Whn rnu rats between day 33 and 85 of life; and (2) cells were transferred from diabetes-resistant LEW.1AR1 or diabetic LEW.1AR1-iddm donors into prediabetic LEW.1AR1-iddm rats at day 30 of life. LEW.1AR1-Whn rnu and prediabetic LEW.1AR1-iddm rats received the following concanavalin A-stimulated immune cells: (1) CD4+ T cells (5 × 106 cells); (2) CD8+ T cells (2.5 × 106 cells); and (3) CD4+ T cells (5 × 106 cells) combined with CD8+ T cells (2.5 × 106 cells).
Blood glucose monitoring
Blood glucose concentrations were determined twice each week [6]. Rats were killed after diagnosis of diabetes defined by two consecutive blood glucose measurements >7.5 mmol/l. There was no sex preference in the diabetes incidence. After adoptive transfer, normoglycaemic animals were killed at day 120.
Morphology of pancreas, pancreas-draining peripheral lymph nodes and spleen
Pancreas and the secondary lymphoid organs were removed, fixed and immunostained by the avidin–biotin complex method or fluorescence method by light microscopy as previously described [1]. The following polyclonal and monoclonal antibodies were used to identify the beta cells and immune cells: rat insulin (A565, 1:500; Dako, Hamburg, Germany); macrophages (ED1; MCA 341), B cells (IgD, 189), pan-T cells (R 73, MCA 453), CD4+ T cells (MCA 55), CD8+ T cells (β-chain, MCA 938,), IL-2R (α-chain, CD25, MCA 494), NK cells (CD 161, MCA 1427), IL-1ß, TNF-α, IFN-γ (all from Serotec); IL-4, IL-10 (Tebu-bio, Offenbach, Germany); TGF-β2 (Santa Cruz Biotechnology, Santa Cruz, CA, USA); and the forkhead box protein 3 (FOXP3) antibody (Natutec, Frankfurt, Germany). Islet infiltration was graded on a score of 0 to 4. The insulitis score was defined as: 0 no infiltration; 1 infiltration restricted to the islet periphery; 2 weak infiltration of the whole islet; 3 severe infiltration of the whole islet; and 4 end-stage islet without beta cells [2]. A mean insulitis score was calculated from ten to 15 islets analysed per pancreas. Cell densities of CD4+ and CD8+ T cells co-expressing IL-4, IL-10 or TGF-ß were quantified by morphometric analysis of immunostained sections using cell P software (Olympus Optical, Hamburg, Germany).
In situ RT-PCR
Sections from the pancreas-draining lymph nodes fixed on three-chamber slides (SuperFrost Plus; Fisher Scientific, Schwerte, Germany) were subjected to in situ RT-PCR gene expression analysis using a two-step protocol with reverse transcription and PCR amplification on a specific thermal cycler (PTC-200 Twin Tower DNA Engine; MJ Research, Waltham, MA, USA) as described before [14]. The primer sequences are listed in Electronic supplementary material (ESM) Table 1. In order to verify the changes in the densities of cytokine gene expression in the immune cells of the pancreas-draining lymph nodes, the amplified mRNA transcripts were densitometrically quantified by a computer-assisted method (cell P software, Olympus) using bright field illumination with the BX61 upright microscope (Olympus). The grey values of gene expression ranged between 35 and 150 for all cytokines. For each sample gene expression data were expressed as integrated grey values/µm2 lymph node area from four optical fields per section.
Statistics
Differences between diabetes incidence were analysed by the log-rank test assuming a level of 0.05 as significant. All other quantitative data are presented as mean values ± SE and were analysed by ANOVA plus Bonferroni’s post test using Prism 5 (Graphpad, San Diego, CA, USA).
Results
Effect of T cell subpopulations on diabetes manifestation in athymic nude LEW.1AR1-Whnrnu rats
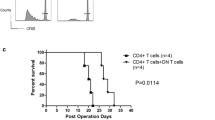
Previous studies have demonstrated the autoaggressive potential of T cells from diabetic LEW.1AR1-iddm rats, which induced insulitis in T cell deficient recipients [6]. To analyse which T cell subpopulation is able to induce diabetes, CD4+ or CD8+ T cell populations were isolated from diabetic LEW.1AR1-iddm rats. T cells (5 × 106 CD4+ T cells, 2.5 × 106 CD8+ T cells) were transferred to each animal in amounts resembling the 2:1 ratio in blood (natural distribution in the LEW.1AR1 strain). Purity of the T cell subpopulations was about 98% before and after stimulation with concanavalin A. FACS analysis also showed that the proportion of CD25+ and FOXP3+ cells remained constant in the range of 2% to 3%, irrespective of whether the donor was diabetic or normoglycaemic. After CD4+ T cell transfer, 50% of the athymic LEW.1AR1-Whn rnu recipients (age 33–85 days) developed diabetes (Table 1). On the other hand, it was not possible to transfer diabetes with 2.5 × 106 (Table 1) or 5 × 106 CD8+ T cells (data not shown) alone. This control experiment was performed to rule out a dose-dependent effect. After combined transfer of CD4+ and CD8+ T cells at a ratio of 2:1, only 10% of the recipients developed diabetes (Table 1). Thus, the diabetes incidence was significantly lower after combined transfer of CD4+ and CD8+ T cells than after transfer of CD4+ T cells alone (Table 1) and manifestation was also delayed (Fig. 1). These data indicate that: (1) diabetes is mainly conferred by transfer of the CD4+ T cell subpopulation; and (2) transfer of the CD8+ T cell subpopulation has a protective potential rather than a diabetogenic effect.
Diabetes manifestation after selective transfer of CD8+ (black asterisk), CD4+ (black circles) or a combination of CD4+ and CD8+ T cells (black squares) from diabetic LEW.1AR1-iddm rats to LEW.1AR1-Whn rnu rats in comparison to control (white triangle). The LEW.1AR1-Whn rnu strain did not develop spontaneous diabetes. The age of the donor rats was between 55 and 65 days, that of all recipients between 33 and 85 days. The T cells were injected i.v. into athymic LEW.1AR1-Whn rnu recipients as described in the Methods. After transfer of T cells, blood glucose of recipients was monitored twice a week. Data were calculated by Maier–Kaplan analyses
All animals with persistent normoglycaemia after adoptive transfer of T cells showed well preserved islets, without signs of immune cell infiltration (not shown). Islets from diabetic LEW.1AR1-Whn rnu recipients after transfer of CD4+ T cells from diabetic LEW.1AR1-iddm donors showed typical signs of infiltration with insulitis scores indistinguishable from islet infiltration in spontaneously diabetic LEW.1AR1-iddm rats (Tables 1 and 2, Fig. 2a–f). Infiltrating cells were identified as macrophages (30–40%) and T cells (55–65%), the latter composed of CD4+ and CD8+ T cell subpopulations at an approximate ratio of 2:1. NK-cells and B cells comprised <10% of infiltrating immune cells. The weak immunostaining of CD8+ T cells in islet infiltrates indicates that this subpopulation is likely to have originated from the LEW.1AR1-Whn rnu recipients (Fig. 2d) [2, 15]. Although the combined transfer of CD4+ and CD8+ T cells from diabetic LEW.1AR1-iddm rats to LEW.1AR1-Whn rnu recipients resulted in a low diabetes incidence of 10%, the islets of the diabetic recipients showed severe insulitis with a score of 3 (Table 1).
Infiltrated pancreatic islets from LEW.1AR1-iddm (a–c) and LEW.1AR1-Whn rnu (d–f) recipients after transfer of CD4+ T cells. LEW.1AR1-iddm rats received CD4+ T cells from diabetes-resistant LEW.1AR1 rats. LEW.1AR1-Whn rnu rats received CD4+ T cells from diabetic LEW.1AR1-iddm rats. Sections were immunostained for cytotoxic CD8+ T cells (a, d), insulin (b, e) and ED1 infiltrating macrophages (c, f). Notably, infiltrating CD8+ T cells in LEW.1AR1-Whn rnu rats (d) showed only a very faint staining for this antigen as a typical sign of immunodeficient status. Magnification × 400, scale bars, 50 μm
Effects of T cell subsets from diabetes-resistant LEW.1AR1 rats on diabetes manifestation in LEW.1AR1-iddm rats
After transfer of 5 × 106 purified CD4+ T cells, 55% of the LEW.1AR1-iddm rats developed diabetes, which was not different from the 60% incidence in the control cohort (Table 2, Fig. 3b). The insulitis score was 2.3 comprising infiltration by CD4+ T cells, CD8+ T cells and macrophages (Table 2, Fig. 2a–c). The transfer of a combination of 2.5 × 106 CD8+ T cells and 5 × 106 CD4+ T cells did not affect the diabetes incidence and insulitis score (Table 2, Fig. 3b). Notably, after transfer of CD8+ T cells alone, the diabetes incidence was significantly decreased to 38% compared with that of spontaneous diabetes in the control cohort without differences in the delay of disease manifestation and insulitis score (Table 2, Fig. 3).
Diabetes manifestation after selective transfer of (a) CD8+ (black asterisk) and (b) CD4+ (black circle) or a combination of CD4+ and CD8+ T cells (black squares) from LEW.1AR1 rats to LEW.1AR1-iddm rats in comparison to control (white triangle). Prediabetic (day 30) LEW.1AR1-iddm rats were used as recipients. LEW.1AR1 donor rats (age >60 days) did not develop spontaneous diabetes. The T cells were injected i.v. into prediabetic LEW.1AR1-iddm recipients as described in the Methods. After transfer of T cells, blood glucose of recipients was monitored twice a week. Data were calculated by Maier–Kaplan analyses
Effects of T cell subsets from diabetic rats on diabetes manifestation in LEW.1AR1-iddm rats
To test the regulatory potential of immune cells after onset of diabetes, we also transferred CD4+ and CD8+ T cells from diabetic LEW.1AR1-iddm rats to prediabetic animals of the same strain. Transfer of the CD4+ population resulted in a diabetes incidence of 60%, which was not different from spontaneous development of diabetes (Table 2, Fig. 4). Rats which received CD8+ T cells showed a significantly (p < 0.05) lower diabetes incidence of 20%. The age of disease manifestation was significantly delayed compared with the control cohort (Table 2, Fig. 4), but the selective transfer of CD8+ T cells did not affect the degree of islet infiltration in diabetic animals. Thus, ex vivo expanded CD8+ T cells from diabetic animals may have a regulatory potential, which is able to confer protection against autoimmune attack.
Diabetes manifestation after selective transfer of CD8+ (black asterisk) or CD4+ (black circle) from diabetic LEW.1AR1-iddm rats to prediabetic LEW.1AR1-iddm rats in comparison to control (black square). Prediabetic (day 30) LEW.1AR1-iddm rats were used as recipients. The T cells were injected i.v. into prediabetic LEW.1AR1-iddm recipients as described in “Methods”. After transfer of T cells, blood glucose of recipients was monitored twice a week. Data were calculated by Maier–Kaplan analyses
CD8+ T cell transfer promotes enrichment of regulatory T cells in pancreas-draining lymph nodes
In the next step we analysed the immune cell repertoire in secondary lymphoid tissues from transferred animals. Pancreas-draining lymph nodes of animals after CD8+ and CD4+ T cell transfer from diabetic donors showed significant differences in the content of CD25+/FOXP3+ regulatory T cells (Tregs), which were not detectable in other peripheral lymph nodes or the spleen. After transfer of CD8+ T cells, pancreas-draining lymph nodes from normoglycaemic LEW.1AR1-iddm rats had a high density of CD25 T cells that were also positive for CD8+ and CD4+ T cells in the paracortical and medullary region (Fig. 5a, b). The cell density per 500 µm2 of CD8+/CD25+ T cells was 56 ± 4 and that of CD4+/CD25+ T cells 33 ± 2 (n = 8). In contrast, rats that developed diabetes after transfer of CD8+ T cells showed low immune cell densities of CD8+/CD25+ T cells (15 ± 2, n = 3) and CD4+/CD25+ T cells (9 ± 1, n = 3) (Fig. 5c, d). Furthermore, transfer of CD4+ T cells also resulted in low density of CD8+/CD25+ T cells (10 ± 1, n = 7) and CD4+/CD25+ T cells (7 ± 1, n = 4) in pancreas-draining lymph nodes in diabetic as well as in non-diabetic animals (n = 3). Additional immunostaining revealed an increase of FOXP3+ T cells after CD8+ T cell transfer to normoglycaemic LEW.1AR1-iddm rats (Fig. 6a, b), as well as after CD4+ T cell transfer; this was not seen in diabetic animals (Fig. 6c, d). Notably, in the spleen and peripheral cervical lymph nodes, FOXP3-positive CD8+/CD25+ and CD4+/CD25+ T cells were only rarely detectable (<5 cells/500 µm2) irrespective of the T cell subpopulation transferred. Thus, transfer of CD8+ T cells resulted selectively in a higher density of Tregs in pancreas-draining lymph nodes of protected normoglycaemic LEW.1AR1-iddm rats.
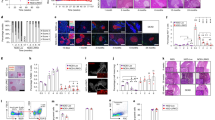
Regulatory T cell subpopulations and cytokine expression in pancreas-draining lymph nodes of protected non-diabetic (a, b, e, f) and diabetic (c, d, g, h) LEW.1AR1-iddm recipients after transfer of CD8+. Prediabetic LEW.1AR1-iddm rats received CD8+ T cells from diabetic LEW.1AR1-iddm rats and protected recipients (a, b, e, f) were compared with non-protected diabetic recipients (c, d, g, h). To identify the Treg subpopulation in CD4+ and CD8+ T cells in overlay modus, sections were immunostained for CD8+ T cells (a, c), CD4+ T cells (b, d) and CD25 (Il-2R) (a–d). Arrows, CD4+/CD25+ T cells (yellow); arrow heads, CD4+/CD25− or CD8+/CD25− T cells (green, Cy2); outlined arrow heads, cells expressing only CD25 (red, Cy3). Gene expression analyses of the proinflammatory cytokine Tnf-α (e, g) and the anti-inflammatory cytokine Il-4 (f, h) were performed by in situ RT-PCR in pancreas-draining lymph nodes. Micrographs are representative of four independent experiments. Magnification (a–d) × 800, (e–h) × 400, scale bars (a), 50 μm, (e) 100 μm
Regulatory FOXP3+ T cell subpopulations in pancreas-draining lymph nodes of protected non-diabetic (a, b) and diabetic (c, d) LEW.1AR1-iddm recipients after transfer of CD8+. Prediabetic LEW.1AR1-iddm rats received CD8+ T cells from diabetic LEW.1AR1-iddm rats and protected recipients (a, b) were compared with non-protected diabetic recipients (c, d). Sections were immunostained for CD8+ T cells (a, c), CD4+ T cells (b, d) and FOXP3 (a–d) to identify the Treg subpopulation in the CD4+ and CD8+ T cells. After CD8+ T cell transfer, pancreas-draining lymph nodes from non-diabetic animals showed an increased density of CD8+/FOXP3+ (a, c) and CD4+/FOXP3+ (b, d) T cells, indicating that Tregs play a protective role in this compartment. In contrast, in diabetic animals, only a few CD4+ or CD8+ T cells were FOXP3-positive, indicating a lower number of Tregs in the pancreas-draining lymph node in the diabetic state. Arrows, CD4+ T cells also FOXP3-positive (yellow-orange); arrow heads, CD4+/FOXP3− or CD8+/FOXP3− T cells (green, Cy2); outlined arrow heads, cells FOXP3-positive only (red, Cy3)
CD8+ T cell transfer induced anti-inflammatory cytokine expression in pancreas-draining lymph nodes
In the next step we investigated whether CD8+ T cell transfer affected the expression pattern of cytokines in pancreas-draining lymph nodes. Non-diabetic animals after CD8+ T cell transfer (Fig. 5e, f) showed a higher gene expression level of the anti-inflammatory cytokines Il4 (Fig. 5f), Tgf-ß (also known as Tgfb1)and Il10 in comparison to diabetic animals, both after CD8+ and CD4+ T cell transfer (Fig. 5h). Gene expression of the pro-inflammatory cytokines Tnf-α (also known as Tnfa; Fig. 5e, g) and Il-1ß (also known as Il1b) showed a reciprocal pattern with low expression levels in non-diabetic rats after CD8+ transfer (Fig. 5e) and high expression levels in diabetic rats after CD8+ transfer (Fig. 5g) and CD4+ transfer. CD8+ T cell transfer resulted in a significant 2.4-fold increase of Il4 and a 1.7-fold increase of Tgf-ß expression in protected normoglycaemic rats (Table 3). In contrast, expression of Il-1ß and Tnf-α was 1.8-fold and 1.5-fold higher in pancreas-draining lymph nodes from diabetic rats than in the protected normoglycaemic cohort after CD8+ T cell transfer (Table 3). The expression pattern indicates that transfer of CD8+ T cells induced a shift away from pro-inflammatory to anti-inflammatory cytokines as potential mediators of the suppressive function of Tregs in pancreas-draining lymph nodes. Here again, cytokine expression in diabetic animals after CD4+ T cell transfer was identical to that in diabetic animals after CD8+ T cell transfer. The density of CD4+ and CD8+ T cells co-expressing IL-4 or TGF-ß was more than fivefold higher in pancreas-draining lymph nodes from normoglycaemic than in those from diabetic LEW.1AR1-iddm rats (Table 3). Overall, the protein levels of anti-inflammatory cytokines correspond well to gene expression data (Table 3). Thus, transfer of CD8+ T cells increased the number of Tregs with both a high level of gene and protein expression for IL-4, TGF-β and IL-10 in pancreas-draining lymph nodes of protected normoglycaemic LEW.1AR1-iddm rats. These findings were independent of whether the CD8+ T cells were derived from the background strain or LEW.1AR1-iddm strain. For comparison, we also analysed pancreas-draining lymph nodes from the donor strains, LEW.1AR1 rats and diabetic LEW.1AR1-iddm rats. Additionally, we performed the same study with normoglycaemic LEW.1AR1-iddm rats, which did not spontaneously develop diabetes (without cell transfer), to clarify whether an anti-inflammatory environment is responsible for protection against autoaggressive cells. Interestingly, and independently of the metabolic state, none of these animals showed a high density (per 500 µm2) of CD4+ (normoglycaemic LEW.1AR1-iddm rats 6 ± 1, diabetic LEW.1AR1-iddm rats 4 ± 1, normoglycaemic LEW.1AR1 rats 4 ± 2) and of CD8+ T cells (normoglycaemic LEW.1AR1-iddm rats 8 ± 2, diabetic LEW.1AR1-iddm rats 6 ± 1, normoglycaemic LEW.1AR1 rats 8 ± 2) that were also positive for CD25+ and FOXP3+ (n = 4 each); nor was significant expression of anti-inflammatory cytokines observed.
Discussion
In the present study we investigated how T cell subpopulations from diabetic and non-diabetic donors affect diabetes incidence in athymic nude LEW.1AR1-Whn rnu and prediabetic LEW.1AR1-iddm recipient rats.
Diabetogenic potential of CD4+ T cells
First, we showed that transfer of concanavalin A-activated CD4+ T cells from diabetic LEW.1AR1-iddm rats could induce diabetes in LEW.1AR1-Whn rnu recipients, while CD8+ T cells subjected to the same protocol failed to do so. Thus, a clear autoaggressive potential of CD4+ T cells was seen, in line with several studies on BB–DP rats and NOD mice [7–12]. The predominant role of CD4+ T cells has been previously demonstrated by selective transfer of CD4+ or CD8+ T cells in immunodeficient NOD strains, whereas CD8+ T cell subsets were not diabetogenic [7, 16]. On the other hand, CD8+ T cells are required for adoptive transfer of the diabetic syndrome in BB–DP rats [11, 17]. The apparent discrepancy between these studies and the LEW.1AR1-iddm rat may be at least in part explained by the lymphopenic background of the BB rat, which could potentially affect the balance between autoaggressive and Treg subtypes. In a previous study, we showed that transfer of concanavalin A-activated total immune cells from spleen and lymph nodes of diabetic LEW.1AR1-iddm rats induced diabetes in 100% of the athymic recipients [6], whereas selective transfer of CD4+ T cells resulted in a diabetes incidence of 50% (Table 1). The lower incidence of diabetes may be explained by the purification process for CD4+ T cells, which do not contain a significant amount of other contributing immune cells including antigen-presenting cells. It should be stressed at this point that the failure of the CD8+ T cell fraction to induce diabetes does not necessarily argue against a pathogenic role of autoaggressive CD8+ T cells in beta cell destruction. Pancreatic islets in diabetic LEW.1AR1-iddm rats are clearly infiltrated by CD8+ T cells and it is likely that activated CD8+ T cells participate in this scenario [2].
Protective role of CD8+ T cells
Somewhat unexpectedly, we found that the mitogen-activated CD8+ T cell fraction was able to reduce diabetes incidence when co-transferred with CD4+ T cells in athymic LEW.1AR1-Whn rnu recipients and also after exclusive transfer in prediabetic LEW.1AR1-iddm rats. The data raise the question of how adoptive transfer of the CD8+ T cell fraction was able to confer protection, a phenomenon that to our knowledge has not been reported so far in the BB–DP rat and NOD mouse models. One possible explanation is that ex vivo stimulation of CD8+ T cells by concanavalin A induced an enrichment of CD8+ regulatory cells in favour of autoaggressive cells. However, FACS analysis of the purified CD8+ subset revealed that after concanavalin A activation the proportion of CD25+ and FoxP3+ cells remained constant in the range of 2–3%, irrespective of whether the donor was diabetic or normoglycaemic. Thus, concanavalin A apparently did not initiate a specific expansion of Tregs. A similar proportion of CD25+/FOXP3+ cells was also observed after mitogenic stimulation of purified CD4+ T cells, which failed to induce any protective effect after adoptive transfer. In spite of these findings, it cannot be ruled out that concanavalin A has a functional effect on the purified T cell subpopulations with regard to the activation state of effector T cells and Tregs. After transfer, purified CD4+ and CD8+ T cell fractions seem to differentially affect the balance between autoaggressive T cells and Tregs in the LEW.1AR1-iddm recipients, thereby determining whether the cells induce diabetes or confer protection against autoimmunity. The protective role observed by us did not interfere with the functional importance of CD8+ T cells for priming events before infiltration and autoreactive destruction of beta cells after islet infiltration [18, 19]. In agreement with findings in NOD mice as well as in BB rats, the immune cell infiltrate in the spontaneous disease development of the LEW.1AR1-iddm rat from start until diabetes manifestation is always composed of macrophages, CD4+ and CD8+ T cells [2]. In the late stage of islet infiltration, a predominance of CD8+ T cells over macrophages was seen [2].
CD8+ T cell transfer confers an anti-inflammatory milieu in pancreas-draining lymph nodes
A principal finding of this study is that transfer of CD8+ T cells led to a higher density of CD25+/FOXP3+ Tregs exclusively in pancreas-draining lymph nodes of protected normoglycaemic LEW.1AR1-iddm rats. There was also a shift towards anti-inflammatory cytokines in this key compartment for immune processes of the pancreas. IL-4, IL-10 and TGF-β have been identified as possible mediators of the postulated regulatory function of Tregs [20–22]. As pancreas-draining lymph nodes of protected normoglycaemic LEW.1AR1-iddm rats also showed a higher density of CD4+/CD25+ T cells, the transfer of CD8+ T cells apparently induced a regulatory phenotype through recruitment of CD4+ Tregs [23]. Overall, this anti-inflammatory environment is likely to inhibit autoaggressive CD4+ T cells and thus autoimmune destruction of beta cells.
There is cumulative evidence that CD8+ T cells may participate as effectors and as regulators in autoimmune diseases [16, 24–28]. A clear inhibitory interaction between regulatory CD8+ and autoreactive CD4+ T cells was found in experimental autoimmune encephalomyelitis [25, 29] and also in human autoimmune diabetes [30–32]. In a mouse model of experimental autoimmune encephalomyelitis, CD8+/CD28− T cells provided protection by inhibiting upregulation of co-stimulatory molecules on antigen-presenting cells, thereby inhibiting activation and clonal expansion of autoaggressive CD4+ cells [26]. Such a scenario could explain why anti-inflammatory cytokine expression and a relative enrichment of Tregs could only be observed in pancreas-draining lymph nodes of LEW.1AR1-iddm rats as the critical compartment for interaction with antigen-presenting cells from islets. Thus, the data allow the hypothesis that CD8+ T cells specifically interact with antigen-presenting cells, thereby initiating a change of the cytokine profile or cell death. Each mechanism would significantly inhibit activation of effector T cells and ultimately the progression of autoimmunity. However, it may be too simplistic to explain the protective effects of CD8+ T cells solely by selective transfer of regulatory subtypes. In this respect, it is important to compare Treg densities and anti-inflammatory cytokine expression in pancreas-draining lymph nodes from normoglycaemic LEW.1AR1-iddm rats with or without transfer of CD8+ T cells. The fact that after CD8+ T cell transfer all normoglycaemic LEW.1AR1-iddm recipients showed a high density of Tregs and expression of anti-inflammatory cytokines indicates that this local anti-inflammatory state is an adaptive response against autoaggressive T cells. Conversely, LEW.1AR1-iddm rats that remained normoglycaemic without transfer of CD8+ or CD4+ T cells had low densities of Tregs and low expression levels of anti-inflammatory cytokines in pancreas-draining lymph nodes. In these rats, the activation state of antigen-presenting cells or autoaggressive T cells may not be high enough to induce beta cell destruction as well as an anti-inflammatory response. The density of Tregs in pancreas-draining lymph nodes probably reflects a three-part adaptive response to T cell transfer: (1) LEW.1AR1-iddm rats that remain normoglycaemic without specific T cell transfer apparently do not develop diabetes due to an insufficient activation of autoaggressive cells rather than to a reactive upregulation of Treg subtypes; (2) after CD4+ T cell transfer, the lack of Treg accumulation in all animals may again result from an insufficient activation of autoaggressive T cells; and (3) only after CD8+ T cell transfer do all normoglycaemic animals show a high density of Tregs and in parallel high anti-inflammatory cytokine expression.
Thus, a dynamic balance between activated autoaggressive T cells and Tregs determines whether a protective anti-inflammatory state will be initiated in pancreas-draining lymph nodes. Overall, our data are in line with studies showing that this lymphatic compartment provides a perfect microenvironment for progression to or protection against autoimmune diabetes [33, 34].
Recent progress in clinical immunomodulation has yielded promising results based on the modulation of anti-CD3 antibodies or upregulation of Tregs [30]. Thus, anti-CD3 treatment induced CD8+/CD25+ T cells that were able to inhibit the response of CD4+ T cells to specific antigens under in vitro conditions [30]. However, our study indicates that the scenario of autoimmune tolerance is more complex with respect both to the role of defined lymphatic compartments, and to a permissive function of CD8+ T cells for induction of Tregs and an anti-inflammatory cytokine state. Additionally, the balance between autoreactive and protective T cell milieu plays an important role in the recurrent autoimmunity in islet transplantation [32]. With this knowledge, it will be possible to refine immunomodulatory strategies at different stages of autoimmunity and thereby control autoaggressive T cells.
Abbreviations
- BB–DP:
-
Biobreeding diabetes-prone
- mAb:
-
Monoclonal antibody
- NK:
-
Natural killer
- Tregs:
-
Regulatory T cells
- FOXP3:
-
Forkhead box protein 3
References
Lenzen S, Tiedge M, Elsner M et al (2001) The LEW.1AR1/Ztm-iddm rat: a new model of spontaneous insulin-dependent diabetes mellitus. Diabetologia 44:1189–1196
Jörns A, Günther A, Hedrich HJ, Wedekind D, Tiedge M, Lenzen S (2005) Immune cell infiltration, cytokine expression, and beta-cell apoptosis during the development of type 1 diabetes in the spontaneously diabetic LEW.1AR1/Ztm-iddm rat. Diabetes 54:2041–2052
Ellerman KE, Like AA (2000) Susceptibility to diabetes is widely distributed in normal class IIu haplotype rats. Diabetologia 43:890–898
Weiss H, Arndt T, Jorns A et al (2008) The mutation of the LEW.1AR1-iddm rat maps to the telomeric end of rat chromosome 1. Mamm Genome 19:292–297
Weiss H, Bleich A, Hedrich HJ et al (2005) Genetic analysis of the LEW.1AR1-iddm rat: an animal model for spontaneous diabetes mellitus. Mamm Genome 16:432–441
Wedekind D, Weiss H, Jorns A, Lenzen S, Tiedge M, Hedrich HJ (2005) Effects of polyinosinic-polycytidylic acid and adoptive transfer of immune cells in the Lew.1AR1-iddm rat and in its coisogenic LEW.1AR1 background strain. Autoimmunity 38:265–275
Christianson SW, Shultz LD, Leiter EH (1993) Adoptive transfer of diabetes into immunodeficient NOD-scid/scid mice. Relative contributions of CD4+ and CD8+ T cells from diabetic vs prediabetic NOD.NON-Thy-1a donors. Diabetes 42:44–55
Logothetopoulos J, Valiquette N, MacGregor D, Hsia T (1987) Adoptive transfer of insulitis and diabetes in neonates of diabetes-prone and -resistant rats. Tissue localization of injected blasts. Diabetes 36:1116–1123
McKeever U, Mordes JP, Greiner DL et al (1990) Adoptive transfer of autoimmune diabetes and thyroiditis to athymic rats. Proc Natl Acad Sci U S A 87:7618–7622
Metroz-Dayer MD, Mouland A, Brideau C, Duhamel D, Poussier P (1990) Adoptive transfer of diabetes in BB rats induced by CD4 T lymphocytes. Diabetes 39:928–932
Whalen BJ, Greiner DL, Mordes JP, Rossini AA (1994) Adoptive transfer of autoimmune diabetes mellitus to athymic rats: synergy of CD4+ and CD8+ T cells and prevention by RT6+ T cells. J Autoimmun 7:819–831
Wicker LS, Miller BJ, Mullen Y (1986) Transfer of autoimmune diabetes mellitus with splenocytes from nonobese diabetic (NOD) mice. Diabetes 35:855–860
Miltenyi S, Muller W, Weichel W, Radbruch A (1990) High gradient magnetic cell separation with MACS. Cytometry 11:231–238
Jörns A, Rath KJ, Bock O, Lenzen S (2006) Beta cell death in hyperglycaemic Psammomys obesus is not cytokine-mediated. Diabetologia 49:2704–2712
Rolstad B, Fossum S (1990) Non-adaptive cellular immune responses as studied in euthymic and athymic nude rats. Spontaneous rejection of allogeneic lymphoid cell grafts by natural killer (NK) cells. Anat Embryol 181:215–226
Yagi H, Matsumoto M, Kunimoto K, Kawaguchi J, Makino S, Harada M (1992) Analysis of the roles of CD4+ and CD8+ T cells in autoimmune diabetes of NOD mice using transfer to NOD athymic nude mice. Eur J Immunol 22:2387–2393
Edouard P, Hiserodt JC, Plamondon C, Poussier P (1993) CD8+ T cells are required for adoptive transfer of the BB rat diabetic syndrome. Diabetes 42:390–397
Anderson MS, Bluestone JA (2005) The NOD mouse: a model of immune dysregulation. Annu Rev Immunol 23:447–485
Lipes MA, Rosenzweig A, Tan KN et al (1993) Progression to diabetes in nonobese diabetic (NOD) mice with transgenic T cell receptors. Science 259:1165–1169
Jaeckel E, Kretschmer K, Apostolou I, von Boehmer H (2006) Instruction of Treg commitment in peripheral T cells is suited to reverse autoimmunity. Semin Immunol 18:89–92
Jaeckel E, Mpofu N, Saal N, Manns MP (2008) Role of regulatory T cells for the treatment of type 1 diabetes mellitus. Horm Metab Res 40:126–136
Kretschmer K, Apostolou I, Jaeckel E, Khazaie K, von Boehmer H (2006) Making regulatory T cells with defined antigen specificity: role in autoimmunity and cancer. Immunol Rev 212:163–169
Wong FS, Dayan CM (2008) Regulatory T cells in autoimmune endocrine diseases. Trends Endocrinol Metab 19:292–299
Han G, Shao H, Peng Y et al (2007) Suppressor role of rat CD8+ CD45RClow T cells in experimental autoimmune uveitis (EAU). J Neuroimmunol 183:81–88
Jiang H, Zhang SI, Pernis B (1992) Role of CD8+ T cells in murine experimental allergic encephalomyelitis. Science 256:1213–1215
Najafian N, Chitnis T, Salama AD et al (2003) Regulatory functions of CD8+CD28- T cells in an autoimmune disease model. J Clin Invest 112:1037–1048
Sempe P, Ezine S, Marvel J et al (1993) Role of CD4+CD45RA+ T cells in the development of autoimmune diabetes in the non-obese diabetic (NOD) mouse. Int Immunol 5:479–489
Xystrakis E, Dejean AS, Bernard I et al (2004) Identification of a novel natural regulatory CD8 T cell subset and analysis of its mechanism of regulation. Blood 104:3294–3301
Jiang H, Kashleva H, Xu LX et al (1998) T cell vaccination induces T cell receptor Vbeta-specific Qa-1-restricted regulatory CD8(+) T cells. Proc Natl Acad Sci U S A 95:4533–4537
Bisikirska B, Colgan J, Luban J, Bluestone JA, Herold KC (2005) TCR stimulation with modified anti-CD3 mAb expands CD8+ T cell population and induces CD8+CD25+ Tregs. J Clin Invest 115:2904–2913
James EA, Kwok WW (2007) CD8+ suppressor-mediated regulation of human CD4+ T cell responses to glutamic acid decarboxylase 65. Eur J Immunol 37:78–86
Huurman VA, Hilbrands R, Pinkse GG et al (2008) Cellular islet autoimmunity associates with clinical outcome of islet cell transplantation. PLoS ONE 3:e2435
Hoglund P, Mintern J, Waltzinger C, Heath W, Benoist C, Mathis D (1999) Initiation of autoimmune diabetes by developmentally regulated presentation of islet cell antigens in the pancreatic lymph nodes. J Exp Med 189:331–339
Turley SJ, Lee JW, Dutton-Swain N, Mathis D, Benoist C (2005) Endocrine self and gut non-self intersect in the pancreatic lymph nodes. Proc Natl Acad Sci U S A 102:17729–17733
Acknowledgements
We gratefully acknowledge the skilful technical assistance of S. Eghtessadi, D. Lischke and U. Sommerfeld. T. Arndt was a graduate student in the Graduate Research Training Programme number 705-2 funded by the Deutsche Forschungsgemeinschaft. This work was supported by NIH grant 1R21AI55464-01, a grant from the Deutsche Forschungsgemeinschaft (JO 395/1-3) and by the European Union (STREP SaveBeta LSHM-CT-2006-036903).
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
T. Arndt and D. Wedekind contributed equally to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Arndt, T., Wedekind, D., Weiss, H. et al. Prevention of spontaneous immune-mediated diabetes development in the LEW.1AR1-iddm rat by selective CD8+ T cell transfer is associated with a cytokine shift in the pancreas-draining lymph nodes. Diabetologia 52, 1381–1390 (2009). https://doi.org/10.1007/s00125-009-1348-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-009-1348-1