Abstract
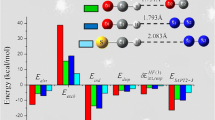
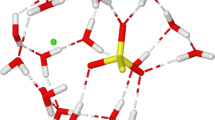
Quantum mechanical methods have been applied to thecis-ONOO−-H2O,cis-ONOO−-(H2O)2 andtrans- ONOO−-H2O complexes. Equilibrium geometries, binding energies, net atomic charges and vibrational frequencies are presented for several different arrangements. The MØller-Plessett second-order perturbation (MP2) method predicted shorter hydrogen bonds than the SCF method, but the computed Hartree-Fock (HF) binding energies are similar to counterpoise corrected MP2 values. The geometry changes of ONOO− and water after solvation are examined. The ONOO− and H2O bond length changes follow typical hydrogen bond structural trends, whereas bond angles in ONOO− are unaffected when the hydrogen bond is formed, similar to the conclusions from NO −2 -(H2O) n HF/6-31G studies and Monte Carlo simulations. Thecis-ONOO−-(H2O) n frequencies are compared with the solution Raman spectrum and with calculations on isolated ONOO−.
Similar content being viewed by others
References
Beckman, J. S.; Beckman, T. W.; Chen, J.; Marshall, P. M.; Freeman, B. A.Proc. Natl. Acad. Sci. (USA) 1990,87, 1620.
King, P. A.; Anderson, V. E.; Edwards, J. O.; Gustafson, G.; Plumb, R. C.; Suggs, J. W.J. Am. Chem. Soc. 1992,114, 5430.
Koppenol, W. H.; Moreno, J. J.; Pryor, W. A.; Ischiropoulos, H.; Beckman, J. S.Chem. Res. Toxicol. 1992,5, 834.
Crow, J. P.; Spruell, C.; Chen, J.; Gunn, C.; Ischiropoulos, H.; Tsai, M.; Smith, C. D.; Radi, R.; Koppenol, W. H.; Beckman, J. S.Free Radical Biol. Med. 1994,16, 331.
Beckan, J. S.; Ichiropoulos, H.; Zhu, L.; van der Woerd, M.; Smith, C.; Chen, J.; Harrison, J.; Martin, J. C.; Tsai, M.Arch. Biochem. Biophys. 1992,298, 438.
Hughes, M. N.; Nicklin, H. G.J. Chem. Soc. (A) 1968, 450.
Shen, M.; Xie, Y.; Schaefer III, H. F.; Deakyne, C. A.J. Chem. Phys. 1990,93, 3379.
Koppenol, W. H.; Klasinc, L.Int. J. Quantum Chem. 1993,20, 1.
Krauss, M.Chem. Phys. Lett. 1994,222, 513.
Tsai, J.-H. M.; Harrison, J. G.; Martin, J. C.; Hamilton, T. P.; van der Woerd, M.; Jablonsky, M. J.; Beckman, J. S.J. Am. Chem. Soc. 1994,116, 4115.
Hamilton, T. P.; Tsai, H.-H.; Tsai, J.-H., van der Woerd, M.; Harrison. J. G.; Jablonsky, M. J.; Beckman, J. S., submitted toJ. Phys. Chem.
Krishnan, R.; Binkley, J. S.; Seeger, R.; Pople, J. A.J. Chem. Phys. 1980,72, 650.
Clark, T.; Chandresekhar, J.; Spitznagel, G. W.; Schleyer, P. v. R.J. Comp. Chem. 1983,4, 294; Frisch, M. J.; Pople, J. A.; Binkley, J. S.J. Chem. Phys. 1984,80, 3265.
Bartlett, R. J.; Silver, D. M.J. Chem. Phys. 1975,62, 3258.
MØller, C.; Plesset, M. S.Phys. Rev. 1934,46, 618.
Pulay, P.Mol. Phys. 1969,17, 197.
Pople, J. A.; Krishnan, R.; Schlegel, H. B.; Binkley, J. S.Int. J. Quant. Chem. 1979,S13, 325.
Handy, N. C.; Amas, R. D.; Gaw, J. F.; Rice, J. E.; Simandiras, E. D.CPL 1985,120, 151
Pople, J. A.; Krishnan, R.; Schlegel, H. B.; Binkley, J. S.Int. J. Quant. Chem. 1979,S13, 325.
Boys, S. F.; Bernardi, F.Mol. Phys. 1970,19, 558.
Hobza, P.; Zahradnik, R.Chem. Rev. 1988,88, 871.
Frisch, M. J.; Del Bene, J. E.; Binkley, J. S.; Schaefer III, H. F.J. Chem. Phys. 1986,84, 2279.
Schwenke, D. W.; Truhlar, D. G.J. Chem. Phys. 1985,82, 2418.
Gaussian 92/DFT, Revision G.2, M. J. Frisch, G. W. Trucks, H. B. Schlegel, P. M. W. Gill, B. G. Johnson, M. W. Wong, J. B. Foresman, M.A. Robb, M. Head-Gordon, E. S. Replogle, R. Gomperts, J. L. Andres, K. Raghavachari, J. S. Binkley, C. Gonzalez, R. L. Martin, D. J. Fox, D. J. Defrees, J. Baker, J. J. P. Stewart, and J. A. Pople, Gaussian, Inc., Pittsburgh, PA, 1993.
Huheey, J. E.; Keiter, E. A.; Keiter, R. L.Inorganic Chemistry, 4th ed.; HarperCollins: New York, 1993.
Beyer, A.; Karpfen, A.; Schuster, P.Topics in Current Chemistry, Vol. 120.Hydrogen Bonds; Springer-Verlag: Berlin, 1984; p 1.
Vinogradov, S. N.; Linnell, R. H.Hydrogen Bonding; Van Nostrand Reinhold: New York, 1971; p 178.
Howell, J. M.; Sapse, A. M.; Singman, E.; Snyder, G.J. Phys. Chem. 1982,86, 2345.
Banerjee, A.; Shepard, R.; Simons, J.J. Chem. Phys. 1980,73, 1814.
Chakrovorty, S. J.; Davidson, E. R.J. Phys. Chem. 1993,97, 6373.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tsai, HH., Hamilton, T.P., Tsai, JH.M. et al. Ab initio studies of peroxynitrite anion-water complexes. Struct Chem 6, 323–332 (1995). https://doi.org/10.1007/BF02293126
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02293126