Summary
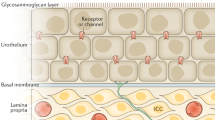
Treatment of superficial bladder cancers by intravesical mitomycin C (MMC) chemotherapy gives a varying and incomplete response. Our recent pharmacokinetics and pharmacodynamics studies have shown that treatment effectiveness is limited by drug degradation in acidic urine and by drug dilution due to residual urine volume and urine production. A model was developed to predict drug exposure in tumors in the bladder wall and to correlate drug exposure with antitumor effect. The model is based on the known pharmacokinetic data in patients treated with intravesical chemotherapy, drug-penetration data in the bladder wall of patients undergoing radical cystectomy, and pharmacodynamic data on patient's bladder-tumor chemosensitivity. Computer simulations based on the model were generated. The simulations predicted that changes in treatment parameters would affect the therapeutic outcome in the following rank order: dose>residual volume>urine production>dosing volume>urine pH>dwell time. Tissue exposure could be enhanced by increased dose, complete bladder emptying, reduced fluid intake, use of the minimal dosing volume, and alkalinization of the urine to a neutral pH. Increasing the dwell time from 2 to 4 h gave an insignificant improvement and posed a compliance problem. The selected optimized regimen of a 40-mg dose, no residual volume, 0.62-ml/min urine production, a 20-ml dosing volume, and alkaline urine pH yielded a calculated 8.5-fold increase in tissue exposure over that achieved by the standard regimen, which consisted of a 20-mg dose, 32-ml residual volume, 1.5-ml/min urine production, a 20-ml dosing volume, and acidic urine pH. On the basis of previously established pharmacodynamic data, we hypothesize that the increase in tissue exposure in the optimized treatment would result in a 20% improvement over the standard therapy along with an increase in the recurrence-free rate from 56% to 76% of patients. A phase III efficacy trial comparing the optimized and standard regimens is proposed.
Similar content being viewed by others
Abbreviations
- MMC:
-
mitomycin C
- SWOG:
-
Southwest Oncology Group
- Cu :
-
urine concentration
- Vu :
-
urine volume
- V0 :
-
dosing volume
- k0 :
-
urine production rate
- Vres :
-
residual volume
- ka :
-
absorption rate constant
- kd :
-
degradation rate constant
- AUC:
-
area under the concentration-time curve; w1/2 haft-width
- Cn x T:
-
drug exposure
- LI:
-
labeling index
- Tinst :
-
instillation time
References
Awadzi K, Adjepon-Yomoah KK, Edwards G, Orme ML'E, Breckenridge AM, Gillies HM (1986) The effect of moderate urine alkalinisation on low dose diethylcarbamazine therapy in patients with onchocerciasis. Br J Clin Pharmacol 21:669
Boring CC, Squires TS, Tong T (1991) Cancer statistics, 1991. CA 41:19
Bracken RB, Johnson DE, Eschenbach AC von, Swanson DA, Furia D de, Crooke S (1980) Role of intravesical mitomycin-C in management of superficial bladder tumors. Urology 16:11
Burdette WJ, Gehan EA (1970) Planning an analysis of clinical studies. C.C. Thomas, Springfield, Illinois, p 35
Cochran WG, Cox GM (1957) Experimental designs. J. Wiley, New York, p 611
Dalton JT, Wientjes MG, Badalament RA, Drago JR, Au JL-S (1991) Pharmacokinetics of intravesical mitomycin C in superficial bladder cancer patients. Cancer Res 51:5144
De Bruijn EA, Van Oosterom AT (1990) Prophylaxis of recurrent superficial bladder cancer: European experience with intravesical mitomycin A (monograph). Bristol-Myers Squibb Co., Evansville, Indiana
Dedrick RL, Flessner MF, Collins JM, Schultz JS (1982) Is the peritoneum a membrane? Am Soc Artificial Int Org J 5:1
Engelmann U, Burger RA, Rumpelt JH, Jacobi GH (1983) Adriamycin permeability of the rat bladder under different conditions. J Urol 129:862
Flessner MF, Dedrick RL, Schultz JS (1984) A distributed model of peritoneal-plasma transport: theoretical considerations. Am J Physiol 246:R597
Flessner MF, Dedrick RL, Schultz JS (1985) A distributed model of peritoneal-plasma transport: analysis of experimental data in the rat. Am J Physiol 248:F413
Flessner MF, Dedrick RL, Schultz JS (1985) A distributed model of peritoneal-plasma transport: tissue concentration gradients. Am J Physiol 248:F425
Groos E, Walker L, Masters JRW (1986) Intravesical therapy studies on the relationship between pH and cytotoxicity. Cancer 58:1199
Herr HW (1987) Intravesical therapy, a critical review. Urol Clin North Am 14:399
Huland H, Otto U (1983) Mitomycin instillation to prevent recurrence of superficial bladder carcinoma. Eur Urol 9:84
Karkkainen S, Neuvonen PJ (1986) Pharmacokinetics of amitriptyline influenced by oral charcoal and urine pH. Int J Clin Pharmacol Ther Toxicol 24:326
Kim HH, Lee G (1989) Intravesical mitomycin C instillation as a prophylactic treatment of superficial bladder tumor. J Urol 141:1337
Kroyer K, Buelow J, Nielsen SL, Kromann-Andersen B (1990) Urinary bladder blood flow: I. Comparison of clearance of locally injected99mTechnetium pertechnate and radioactive microsphere technique in dogs. Urol Res 18:223
Lum BL, Torti FM (1991) Adjuvant intravesical pharmacotherapy for superficial bladder cancer. J Natl Cancer Inst 83:682
Nemeth CJ, Khan RM, Kichner P, Adams R (1977) Changes in canine bladder perfusion with distension. Invest Urol 15:149
Niijima T, Koiso K, Akaza H, the Japanese Urological Cancer Research Group for Adriamycin (1983) Randomized clinical trial on chemoprophylaxis of recurrence in cases of superficial bladder cancer. Cancer Chemother Pharmacol 11:S79
Richie JP, Shipley WU, Yagoda A (1989) Cancer of the bladder. In: Devita V Jr, Hellman S, Rosenberg SA (eds) Cancer: principles and practice of oncology, J.B. Lippincott, Philadelphia, p 1008
Schmittgen TD, Wientjes MG, Badalament RA, Au JL-S (1991) Pharmacodynamics of mitomycin C in cultured human bladder tumors. Cancer Res 51:3849
Soloway MS (1988) Intravesical therapy for bladder cancer. Urol Clin North Am 15:661
Tolley DA, Hargreave TB, Smith PH, Williams JL, Grigor KM, Parmar PKB, Freedman LS, Uscinska BM (1988) Effect of intravesical mitomycin C on recurrence of newly diagnosed superficial bladder cancer: interim report from the Medical Research Council subgroup on superficial bladder cancer (Urologic Cancer Working Party). BMJ 296:1759
Watanabe H, Nako M, Nakagawa S, Takada H (1987) Size and location of tumors influencing the effect of bladder instillation therapy. Cancer Chemother Pharmacol 20:S49
Wientjes MG, Dalton JT, Badalament RA, Drago JR, Au JL-S (1991) A method to study drug concentration-depth profiles in tissues: mitomycin C in dog bladder wall. Pharm Res 8:168
Wientjes MG, Dalton JT, Badalament RA, Drago JR, Au JL-S (1991) Bladder wall penetration of mitomycin C after intravesical instillation in dogs. Cancer Res 51:4347
Wientjes MG, Au JL-S, Badalament RA, Drago JR (1991) Bladder tissue penetration of mitomycin C in patients receiving intravesical therapy. Proc Am Assoc Cancer Res 32:178
Wientjes MG, Badalament RA, Hassan F, Drago JR, Au JL-S (1992) Factors influencing bladder wall concentrations during intravesical cancer chemotherapy. Proc Am Assoc Cancer Res 33:556
Yamaoka K, Nakagawa T, Uno T (1978) Application of Akaike's information criterion (AIC) in the evaluation of linear pharmacokinetic equations. J Pharmacokinet Biopharm 6:165
Zimmerman CL, O'Connell MB, Soria I (1990) The effects of urine pH modification on the pharmacokinetics and pharmacodynamics of phenylpropanolamine. Pharm Res 7:96
Zingg EJ, Wallace DMA (1985) The treatment of superficial bladder tumors. In: Zingg EJ, Wallace DMA (eds) Bladder Cancer. Springer, Berlin Heidelberg New York, p 161
Author information
Authors and Affiliations
Additional information
This work was partly supported by MERIT grant R37 CA-49816 and Research Career Development Award K04 CA-01497 (to J. L.-S. A.) from the National Cancer Institute, DHHS
Rights and permissions
About this article
Cite this article
Guillaume Wientjes, M., Badalament, R.A. & Au, J.L.S. Use of pharmacologic data and computer simulations to design an efficacy trial of intravesical mitomycin C therapy for superficial bladder cancer. Cancer Chemother. Pharmacol. 32, 255–262 (1993). https://doi.org/10.1007/BF00686169
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00686169