Summary
Nitroglycerin-(NTG)-induced headache and dilatation of the radial artery were followed in a double blind, randomized, placebo-controlled, cross-over study in 6 healthy volunteers. NTG 0.5 μg · kg−1 · min−1 or saline were infused IV for 7 h, and subsequently the infusion rate was doubled for 10 min. The radial artery diameter was measured repeatedly with high frequency ultrasound and pain was scored using a 10 point verbal scale.
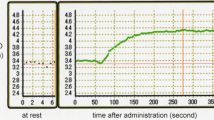
After 5 min of NTG infusion both headache and the arterial diameter differed significantly from baseline, and no further significant change occurred. The intensity of the headache was mild to medium (median headache score 3, range 1–7). The mean dilatation of the radial artery was 36%. The dilatation in each individual, was stable over time, both during NTG and placebo, and it did not change with the double infusion rate. The headache score in each individual was more fluctuan.
No tolerance either to the NTG-induced headache or arterial dilatation was observed.
Similar content being viewed by others
References
Iversen HK, Olesen J, Tfelt-Hansen P (1989) Intravenous nitroglycerin as an experimental headache model. Basic characteristics. Pain 38: 17–24
Iversen HK, Nielsen TH, Garre K, Tfelt-Hansen P, Olesen J (1992) Dose-dependent headache response and dilatation of extremity and extracranial arteries after three doses of 5-isosor-bidemononitrate. Eur J Clin Pharm 42: 31–35
Iversen HK (1992) N-acetylcysteine enhances nitroglycerin-induced headache and cranial arterial responses. Clin Pharmacol Ther 52: 125–133
Fletcher A, McLoone P, Bulpitt C (1988) Quality of life on angina therapy: a randomised controlled trial of transdermal glyceryl trinitrate against placebo. Lancet II: 4–8
Dellborg M, Gustafsson G, Swedberg K (1991) Buccal versus intra-venous nitroglycerin in unstable angina pectoris. Eur J Clin Pharmacol 41: 5–9
Zimrin D, Reichek N, Bogin K, Cameron S, Douglas P, Fung HL (1985) Antianginal effects of i.v. nitroglycerin. Circulation 72 [Suppl 3]: 460
Roth A, Kulick D, Freidenberger L, Hong R, Rahimtoola SH, Elkayam U (1987) Early tolerance to hemodynamic effects of high dose transdermal nitroglycerin in responders with severe chronic heart failure. J Am Coll Cardiol 4: 858–864
May DC, Popma JJ, Black WH, Schaefer S, Lee HR, Levine BD, Hillis LD (1987) In vivo induction and reversal of nitroglycerin tolerance in human coronary arteries. N Engl J Med 13 805–809
Nielsen TH, Iversen HK, Tfelt-Hansen P (1990) Determination of the luminal diameter of the radial artery in man by high frequency ultrasound. A methodological study. Ultrasound Med Biol 8: 787–791
Baaske DM, Amann AH, Karnatz NN, Wong J, Wagenknecht DM, Carter JE, Stoll RG (1982) Administration set suitable for use with intravenous nitroglycerin. Am J Hosp Pharm 39: 121–122
Matthews JNS, Altman DG, Cambell MJ, Royston P (1990) Analysis of serial measurements in medical research. Br Med J 300: 230–235
Andersen B, Holm P (1984) Problems with P. Significance testing in medical research. Roche, Denmark, pp 63–72
Leier CV, Huss P, Magorien RD, Unverferth DV (1983) Improved exercise capacity and differing arterial and venous tolerance during chronic isosorbid dinitrate therapy for congestive heart failure. Circulation 67: 817–822
Noonan PK, Benet LZ (1987) Variable glyceryl dinitrate formation as a function of route of nitroglycerin administration. Clin Pharm Ther 3: 273–277
Bogaert MG, Rosseel MT, De Schaepdryver AF (1968) Cardiovascular effects of glyceryldinitrates as compared to glyceryltrinitrate. Arch Int Pharmacodyn 2: 458–460
Nyberg G, Westling H (1981) Circulatory effects of sublingual and oral sustained release nitroglycerin in healthy young men. Eur J Clin Pharmacol 19: 245–249
Imhof PR, Sieber A, Hodler J, Müller P, Ott B, Fankhausen P, Chu L-C, Cérardin A (1982) Plasma concentrations and haemody-namic effects of nitroglycerin during and after intravenous infusion in health volunteers Eur J Clin Pharmacol 23:99–106
Dalsgaard-Nielsen T (1955) Migraine diagnostics with special reference to pharmacological tests. Int Arch All 7: 312–322
Ekbom K (1968) Nitroglycerin as a provocative agent in cluster headache. Arch Neurol 19: 487–493
Hansen H, Drewes M (1970) The nitroglycerin ointment test — a double-blind examination. Danish Med Bull (1970) 17: 226–229
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Iversen, H.K., Nielsen, T.H., Tfelt-Hansen, P. et al. Lack of tolerance of headache and radial artery diameter during a 7 hour intravenous infusion of nitroglycerin. Eur J Clin Pharmacol 44, 47–50 (1993). https://doi.org/10.1007/BF00315279
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00315279