Abstract
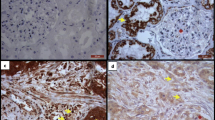
Gelatinase B (92 kD matrix metalloproteinase-9, MMP-9), an enzyme capable of degrading several connective tissue components, was demonstrated by immunolocalization in all specimens of colorectal carcinoma (n=40), but its distribution between specimens was variable. MMP-9 expression was more frequently observed in advanced tumour stages and was especially prevalent at the side and deep margins of the tumours, and ulceration sites. MMP-9 staining was observed for transformed epithelial cells, macrophages and neutrophils, but seldom for vascular or stromal cells. By contrast, the enzyme was absent from epithelial cells of normal mucosal tissue. Immunostaining of type IV collagen, the major structural component of basement membranes, revealed a general depletion or loss of these structures both within the tumours and at the tumour margins. Dual immunolocalization of MMP-9 and type IV collagen demonstrated that MMP-9 expression at specific sites in the tumour was often inversely related to the distribution of type IV collagen MMP-9 expression was most pronounced at the invasive tumour margins and in microfoci where tumour cells were in close proximity to inflammatory cells. Such observations support the concept that localized proteolytic and collagenolytic activities contribute to the invasive properties of colorectal tumours.
Résumé
La gélatinase B (92 kD, matrice métalloprotéinase-9, MMP-9), une enzyme capable de dégrader de nombreux composants du tissu conjonctif a été mise en évidence par immuno-localisation dans des specimens de cancers colo-rectaux (n=40) mais sa distribution entre les divers échantillons était inégale. La mise en évidence de MMP-9 était plus fréquemment observée dans des tumeurs d'un stade avancé et particulièrement sur les berges et en profondeur de ces tumeurs et dans les zones ulcérées. La coloration du MMP-9 a été observée dans des cellules épithéliales transformées, des macrophages et des neutrophiles mais rarement dans des cellules vasculaires ou des cellules du stroma. Au contraire l'enzyme était absente des cellules épithéliales d'une muquese normale. L'immuno-coloration du collagène type IV, le composant structurel majeur des membranes basales, montrent une déplétion générale ou une perte de ces structures à la fois dans les tumeurs et dans les marges de celles-ci. La localisation par immuno-histochimie à la fois de MMP-9 et du type IV du collagène démontre que l'expression du MMP-9 dans certains sites tumoraux est souvent en relation inverse à la distribution du collagène de type IV. L'expression de MMP-9 était plus prononcée sur les berges d'une tumeur invasive et dans des microfoyers tumouraux lorsque les cellules tumorales étaient à proximité immédiate de cellules inflammatoires. Ces observations soutiennent le concept que des activités protéolytiques et collagénolytiques localisées contribuent à l'extansion des tumeurs colo-rectales.
Similar content being viewed by others
References
Dukes CE, Bussey HJR (1958) The spread of the rectal cancer and its effect on prognosis. Br J Surg 12:309–320
Jass JR, Atkin WS, Cuzick J, Bussey HJR, Morson BC, Northover JMA, Todd IP (1986) The grading of rectal cancer: historical perspectives and multivariate analysis of 447 cases. Histopathology 10:437–459
Jass JR, Love SB, Northover JMA (1987) A new prognostic classification of rectal cancer. Lancet i:1303–1306
Hase K, Shatney C, Johnson D, Trollope M, Vierra M (1993) Prognostic value of tumour “budding” in patients with colorectal cancer. Dis Colon Rectum 36:627–635
Vracko R (1974) Basal lamina scaffold — anatomy and significance for maintenance of orderly tissue structure. Am J Pathol 77:314–346
Abrahamson DR (1986) Recent studies on the structure and pathology of basement membranes. J Pathol 149:257–278
Albrechtsen R, Wewer UM, Liotta LA (1986) Basement membranes in human cancer. Pathol Annu 21:251–276
Forster SJ, Talbot IC, Clayton DG, Critchley DR (1986) Tumour basement membrane laminin in adenocarcinoma of rectum: An immunohistochemical study of biological and clinical significance. Int J Cancer 37:813–817
Grigioni WF, Biagni G, Errico AD, Milani M, VillanacciV, Garbisa S, Mattioli S, Gozzetti C, Mancini AM (1986) Behaviour of basement membrane antigens in gastric and colorectal cancer. Acta Pathol Jpn 36:173–184
Hewitt RE, Powe DG, Griffin NR, Turner DR (1991) Relationship between epithelial basement membrane staining patterns in primary colorectal carcinomas and the extent of tumour spread. Int J Cancer 48:855–860
Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, Engler JA (1993) Matrix metalloproteinases: A review Crit Rev Oral Biol Med 4:197–250
Murphy G, Reynolds JJ (1993) Extracellular matrix degradation. In: Royce PM, Steinman B (eds) Connective tissue and its heritable disorders: Molecular and genetic aspects, Wiley-Liss Inc, New York, pp 287–316
Nagase H, Barrett AJ, Woessnar JF, Jr (1992) Nomenclature and glossary of the matrix metalloproteinases. In: Birkedal-Hansen H, Werb Z, Welgus HG, Van Wart HE (eds) Matrix Metalloproteinases and Inhibitors, Gustav Fischer, Stuttgart, pp 421–424
Morodomi T, Ogata Y, Sasaguri Y, Morimatsu M, Nagase H (1992) Purification and characterisation of matrix metalloproteinase-9 from U937 monocytic leukaemia and HT 1080 fibrosarcoma cells. Biochem J 285:603–611
Murphy G, Docherty AJP, Hembry RM, Reynolds JJ (1991) Metalloproteinases and tissue damage. Br J Rheumatol 30 (Suppl 1):25–31
Liotta LA, Rao CN, Barsky SH (1983) Tumour invasion and the extracellular matrix. Lab Invest 49:636–649
Van der Stappen JWJ, Hendriks T, Wobbes T (1990) Correlation between collagenolytic activity and grade of histological differentiation in colorectal tumours. Int J Cancer 45:1071–1078
Hewitt RE, Leach IH, Powe DG, Clark IM, Cawston TE, Turner DR (1991) Distribution of collagenase and tissue inhibitor of metalloproteinases (TIMP) in colorectal tumours. Int J Cancer 49:666–672
Poulsom R, Pignatelli M, Stetler-Stevenson WG, Liotta LA, Wright PA, Jeffrey RE, Longcroft JM, Rogers L, Stamp GWH (1992) Stromal expression of 72 kda type IV collagenase (MMP-2) and TIMP-2 mRNAs in colorectal neoplasia. Am J Pathol 141:389–396
D'Errico A, Garbisa S, LiottaLA, Castronova V, Stetler-Stevenson WG, Grigioni WF (1991) Augmentation of type IV collagenase, laminin receptor, and Ki67 proliferation antigen associated with human colon, gastric, and breast carcinoma progression. Mod Pathol 4:239–246
Ogata Y, Pratta MA, Nagase H, Arner EC (1992) Matrix metalloproteinase-9 (92-kDa gelatinase/type-IV collagenase) is induced in rabbit articular chondrocytes by cotreatment with interleukin 1-beta and a protein kinase C activator. Exp Cell Res 201:245–249
Morson BC, DawsonIMP, Day DW, Jass JR, Price AB, Williams GT (1991) Morson & Dawson's Gastrointestinal Pathology, 3rd edn. Blackwell, Oxford, pp 597–629
Jass JR (1992) Epithelial and mesenchymal tumours of the small and large bowel. In: McGee JO'D, Isaacson PG, Wright NA (eds) Oxford Textbook of Pathology, v 2 a, pp 1258–1281
Burtin P, Chavanel G (1983) Immunofluorescence study of the antigens of the basement membrane and the peritumoral stoma in human colonic adenocarcinomas. Ann NY Acad Sci 420:229–236
Havenith MG, Arends JW, Simon R, Volovics A, Wiggers T, Bosman FT (1988) Type IV collagen immunoreactivity in colorectal cancer. Cancer 62:2207–2211
Barsky SH, Siegal GP, Jannotta F, Liotta LA (1983) Loss of basement membrane components by invasive tumours but not by their benign counterparts. Lab Invest 49:140–147
Nakamura K, Mori M, Enjoi M (1987) Distribution of basement membrane antigens in clinical gastric adenocarcinomas: an immunohistochemical study. J Clin Pathol 40:1418–1423
Nakajima M, Chop AM (1991) Tumour invasion and extracellular matrix degradative enzymes: regulation of activity by organ factors. Semin Cancer Biol 2:115–127
Nagase H, Ogata Y, Suzuki K, Enghild JJ, Salvesen G (1991) Substrate specificities and activation mechanisms of matrix metalloproteinases. Biochem Soc Trans 19:715–718
Senior RM, Griffin GL, Fliszar CJ, Shapiro SD, Goldberg GI, Welgus HG (1991) Human 92-kilodalton and 72-kilodalton type IV collagenases are elasteses. J Biol Chem 266:7870–7875
Woolley DE (1984) Collagenolytic mechanisms in tumour cell invasion. Cancer Metastasis Rev 3:361–372
Dabbous MK, Woolley DE, Hanley L, Carter LM, Nicolson GL (1986) Host-mediated effectors of tumour invasion: role of mast cells in matrix degradation. Clin Exp Metastasis 4:141–152
Garbisa S, D'Errico A, Grigioni WF, Biagni G, Caenazzo C, Fastelli G, Stetler-Stevenson W, Liotta LA (1990) Type IV collagenase augmentation associated with colorectal and gastric cancer progression. In: Harris CC (ed) Genetical Mechanisms in Carcinogenesis and Tumour Progression, Wiley-Liss, Inc., pp 203–212
Pyke C, Ralfkiær E, Tryggvason K, DanøK (1993) Messenger RNA for two type IV collagenases is located in stromal cells in human colon cancer. Am J Pathol 142:359–365
Woessner JF Jr (1991) Matrix metalloproteinases and their inhibitors in connective tissue remodelling. FASEB J 5:2145–2154
Watanabe H, NakanishiI, Yamashita K, Hayakawa T, Okada Y (1993) Matrix metalloproteinase-9 (92 kDa gelatinase/type IV collagenase) from U937 monoblastoid cells: correlation with cellular invasion. J Cell Sci 104:991–999
Juarez J, Clayman G, Nakajima, M, Tanabe KK, Saya H, Nicolson GL (1993) Role and regulation of expression of 92-kDa type-IV collagenase (MMP-9) in 2 invasive squamous-cell-carcinoma cell lines of th eoral cavity. Int J Cancer 55:10–18
BernhardEJ, Muschel RJ, Hughes EN (1990) Mr 92,000 gelatinase release correlates with the metastatic phenotype in transformed rat embryo cells. Cancer Res 50:3872–3877
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jeziorska, M., Haboubi, N.Y., Schofield, P.F. et al. Distribution of gelatinase B (MMP-9) and type IV collagen in colorectal carcinoma. Int J Colorect Dis 9, 141–148 (1994). https://doi.org/10.1007/BF00290191
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00290191