Summary
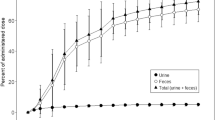
The renal clearance of melphalan and the fraction unbound in plasma were determined after intravenous infusion of 5 mg/m2 over 5 min in nine patients with cancer to obtain information regarding the mechanism of renal handling of melphalan. Four of the patients underwent bone marrow transplantation and also received an IV dose of 220 mg/m2. Total melphalan clearance after the 5 mg/m2 dose ranged from 66.0 to 272 ml/min per m2; the percentage of the dose excreted unchanged in urine, from 2.5% to 92.8%; renal clearance, from 4.1 to 188 ml/min per m2; the fraction unbound in plasma, from 0.0598 to 0.460; and t1/2β, from 39.4 to 84.3 min. Unbound melphalan clearance and renal clearance calculated from the unbound fraction in plasma for each patient ranged from 441 to 3356 ml/min per m2 and 15 to 961 ml/min per m2 respectively and were not related to serum albumin, serum creatinine or creatinine clearance. The percentage of the dose exctreted and melphalan renal clearance were not related to urine flow. There was evidence of active secretion of melphalan in the kidney an possible reabsorption. There were no significant paired differences in melphalan disposition between the high- and low-dose studies. Highly variable renal clearance involving active secretion may contribute in part to large interpatient differences in the total plasma clearance of melphalan in patients with cancer.
Similar content being viewed by others
References
Alberts DS, Chang SY, Chen H-SG, Moon TE, Evans TL, Furner RL, Himmelstein K, Gross JF (1979) Kinetics of intravenous melphalan. Clin Pharmacol Ther 26: 73–80
Ardiet C, Tranchand B, Biron P, Rebattu P, Philip T (1986) Pharmacokinetics of high-dose intravenous melphalan in children and adults with forced diuresis. Cancer Chemother Pharmacol 16: 300–305
Bosanquet AG, Gilby ED (1982) Pharmacokinetics of oral and intravenous melphalan during routine treatment of multiple myeloma. Eur J Cancer Clin Oncol 18: 355–362
Brox L, Birkett L, Belch A (1969) Pharmacology of intravenous melphalan in patients with multiple myeloma. Cancer Treat Rep 6: 27–32
Ehrsson H, Lonroth U (1982) Degradation of melphalan in aqueouse solutions — influence of human albumin binding. J Pharm Sci 7: 826–827
Gouyette A, Hartmann O, Pico J-L (1986) Pharmacokinetics of high-dose melphalan in children and adults. Cancer Chemother Pharmacol 16: 184–189
Greig NH, Sweeney DJ, Rapoport SI (1987) Melphalan concentration dependent plasma protein binding in healthy humans and rats. Eur J Clin Pharmacol 32: 179–185
Ninane J, Baurain R, de Selys A, Trouet A, Cornu G (1985) High dose melphalan in children with advanced malignant disease. Cancer Chemother Pharmacol 15: 263–267
Reece PA, Kotasek D, Morris RG, Dale BM, Sage RE (1986) The effect of food on oral melphalan absorption. Cancer Chemother Pharmacol 16: 194–197
Reece PA, Dale BM, Morris RG, Kotasek D, Gee D, Rogerson S, Sage RE (1987) Effect of L-leucine on oral melphalan kinetics in patients. Cancer Chemother Pharmacol 20: 256–258
Taha IA-K, Ahmad RA, Rogers DW, Pritchard J, Rogers HJ (1983) Pharmacokinetics of melphalan in children following high-dose intravenous injection. Cancer Chemother Pharmacol 10: 212–216
Vistica DT (1983) Cellular pharmacokinetics of the phenylalanine mustards. Pharmacol Ther 22: 379–405
Woodhouse KW, Hamilton P, Lennard A, Rawlins MD (1983) The pharmacokinetics of melphalan in patients with multiple myeloma: an intravenous/oral study using a conventional dose regimen. Eur J Clin Pharmacol 24: 283–285
Author information
Authors and Affiliations
Additional information
This study was supported by a grant from The Queen Elizabeth Hospital Research Foundation
Rights and permissions
About this article
Cite this article
Reece, P.A., Hill, H.S., Green, R.M. et al. Renal clearance and protein binding of melphalan in patients with cancer. Cancer Chemother. Pharmacol. 22, 348–352 (1988). https://doi.org/10.1007/BF00254244
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00254244