Summary
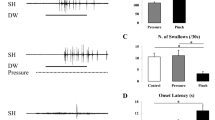
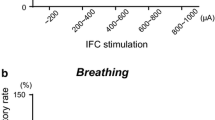
Swallowing is a patterned motor activity generated by neurons located within the nucleus tractus solitarius (NTS). An excitatory amino acid (EAA) neurotransmitter, such as glutamate (GLU), is suspected of being involved in the initiation of swallowing by NTS neuronal components. However, swallowing can still be elicited in animals anesthetized with ketamine, an antagonist of the N-methyl-D-aspartate (NMDA) subclass of EAA receptors. The present experiments were therefore designed to investigate the influence of EAA administration within the NTS on the swallowing motor acitivity of rats anesthetized with ketamine. Pressure microinjections of GLU in doses ranging from 25 to 500 pmol elicited swallowing. This effect was dose-dependent and was not reproduced when control injections of the vehicle solution were performed. Microinjections of the GLU agonists, quisqualate (QUIS) and NMDA, in doses ranging between 2.5 and 50 pmol, also induced swallowing motor activities. QUIS, like GLU, elicited a short series of swallows at a brief latency while NMDA generated long-lasting rhythmic swallowing with a longer latency. Swallowing induced by GLU microinjections (100 pmol) was suppressed almost completely by local pretreatment with either the broad spectrum EAA receptor antagonist, gamma-D-glutamylglycine (250 pmol), or the more selective non-NMDA antagonist, 6-cyano-7-nitroquinoxaline-2,3-dione (50–100 pmol), but not by pretreatment with the selective NMDA antagonist, DL-2-amino-5-phosponovalerate (250 pmol). On the other hand, pretreatment with DL-2-amino-5-phosphonovalerate (50 pmol) suppressed the deglutitions induced by NMDA microinjections (10 pmol) but not those elicited by QUIS microinjections (10 pmol). These results provide evidence that swallowing can be induced by activation of EAA receptors of both the NMDA and the non-NMDA subclasses located within the NTS. Furthermore they indicate that both subclasses may still be active in ketamine-anesthetized animals.
Similar content being viewed by others
References
Altschuler SM, Bao X, Bieger D, Hopkins DA, Miselis RR (1989) Viscerotopic representation of the upper alimentary tract in the rat: sensory ganglia and nuclei of the solitary and spinal trigeminal tracts. J Comp Neurol 283: 248–268
Anis NA, Berry SC, Burton NR, Lodge D (1983) The dissociative anaesthetics, ketamine and phencyclidine, selectively reduce excitation of central mammalian neurones by N-methyl-aspartate. Br J Pharmacol 79: 565–575
Ascher P, Nowak L (1987) Electrophysiological studies of NMDA receptors. Trends Neurosci 10: 284–288
Ascher P (1989) Glutamate receptors and glutamatergic synapses. In: Evangelopoulos AE (eds) Receptors, membrane transport and signal transduction. pringer Berlin Heidelberg, pp 127–146
Carpenter DO (1989) Central nervous system mechanisms in deglutition and emesis. In: Wood JD (eds) The gastrointestinal system I. Handbook of physiology. American Physiological Society, Bethesda, pp 685–714
Cherkaoui N, Jean A, Kessler JP (1988) Activation of excitatory amino acid receptors within the nucleus tractus solitarius elicits the swallowing motor pattern in the anaesthetized rat. J. Physiol (Lond) 407: 16P
Cotman CW, Monaghan DT, Ganong AH (1988) Excitatory amino acid neurotransmission: NMDA receptors and Hebb-type synaptic plasticity. Ann Rev Neurosci 11: 61–80
Doty RW (1968) Neural organization of deglutition. In: Code FC (eds) Alimentary canal. Handbook of physiology, Sect VI, Vol IV. American Physiological Society, Washington, pp 1861–1902
Garthwaite J (1985) Cellular uptake disguises action of L-glutamate on N-methyl-D-aspartate receptors. Br J Pharmacol 85: 297–307
Giorguieff MF, Kemel ML, Glowinski J (1977) Presynaptic effect of L-glutamic acid on the release of dopamine in rat striatal slices. Neurosci Lett 6: 73–77
Grillner S, Wallen P, Dale N, Brodin L, Buchanan J, Hill R (1987) Transmitters, membrane properties and network circuitry in the control of locomotion in lamprey. Trends Neurosci 10: 34–41
Hamilton RB, Norgren R (1984) Central projections of gustatory nerves in the rat. J Comp Neurol 222: 560–577
Hashim MA, Bieger D (1987) Mediation of differential glutamate responses by NMDA and kainate receptors associated with deglutitive neural substrates within the rat solitarius complex. Soc Neurosci Abstr 13: 54.9
Hashim MA, Bieger D (1989) Excitatory amino acid receptormediated activation of solitarial deglutitive loci. Neuropharmacology 28: 913–921
Honey CR, Miljkovic Z, MacDonald JF (1985) Ketamine and phencyclidine cause a voltage-dependent block of responses to 1-aspartic acid. Neurosci Lett 61: 135–159
Honoré T, Davies SN, Drejer J, Fletcher EJ, Jacobsen P, Lodge D, Nielsen FE (1988) Quinoxalinediones: potent competitive non-NMDA glutamate receptor antagonists. Science 241: 701–703
Jean A (1972a) Localisation et activité des neurones déglutiteurs bulbaires. J Physiol (Paris) 64: 227–268
Jean A (1972b) Effets de lésions localisées du bulbe rachidien sur le stade oesophagien de la déglutition. J Physiol (Paris) 64: 507–516
Jean A (1984) Brainstem organization of the swallowing network. Brain Behav Evol 25: 109–116
Jean A, Car A (1979) Inputs to the swallowing medullary neurons from the peripheral afferent fibers and the swallowing cortical area. Brain Res 178: 567–572
Jean A, Cherkaoui N, Kessler JP, Catalin D (1986) Initiation of the complex motor sequence of swallowing by glutamate microinjections within the lateral solitary complex of the medulla oblongata. Neurosci Lett Suppl 26: S 151
Kemp JA, Foster AC, Wong EHF (1987) Non-competitive antagonists of excitatory amino acid receptors. Trends Neurosci 10: 294–298
Kessler JP, Jean A (1985a) Identification of the medullary swallowing regions in the rat. Exp Brain Res 57: 256–263
Kessler JP, Jean A (1985b) Inhibition of the swallowing reflex by local application of serotonergic agents into the nucleus of the solitary tract. Eur J Pharmacol 118: 77–85
Kessler JP, Jean A (1986) Effect of catecholamines on the swallowing reflex after pressure injection into the lateral solitary complex of the medulla oblongata. Brain Res 386: 69–77
Kubo T, Kihara M (1988) Evidence of N-methyl-D-aspartate receptor mediated modulation of the aortic baroreceptor reflex in the rat nucleus tractus solitarii. Neurosci Lett 87: 69–74
Lewis SJ, Verberne AJM, Summers RJ, Beart PM, Cincotta M (1988) Reduced glutamate binding in rat dorsal vagal complex after nodose ganglionectomy. Brain Res Bull 21: 913–915
MacDonald JF, Miljkovic Z, Pennefather P (1987) Use-dependent block of excitatory amino acid currents in cultured neurons by ketamine. J Neurophysiol 58: 251–266
Marco LA, Reed TF, Aides LD, Chronister RB (1988) An electrographic characterization of ketamine-induced linguopharyngeal motor activity. Clin Neuropharmacol 11: 141–150
Marco LA, Joshi RS, Brigham TE, Aides LD, Chronister RB (1989) Effects of clozapine on ketamine-induced linguopharyngeal events in rats. Eur J Pharmacol 164: 171–173
McCaman RE, McKenna DG, Ono JK (1977) A pressure system for intracellular ejections of picoliter volumes. Brain Res 136: 141–147
McLennan H (1984) A comparison of the effects of N-methyl-D-aspartate and quinolinate on central neurones of the rat. Neurosci Lett 46: 157–160
Miller AJ (1982) Deglutition. Physiol Rev 62: 129–184
Monaghan DT, Cotman CW (1985) Distribution of N-methyl-D-aspartate sensitive L-[3H]glutamate-binding sites in rat brain. J Neurosci 5: 2909–2919
Nowak L, Bregestovski P, Ascher P, Herbert A, Prochiantz A (1984) Magnesium gates glutamate-activated channels in mouse central neurones. Nature 307: 462–465
Ottersen OP, Storm-Mathisen JL (1984) Neurons containing or accumulating transmitter amino acids. In: Björklund A, Hökfelt T, Kuhar MJ (eds) Classical transmitters and transmitter receptors in the CNS, Part II. Handbook of chemical neuroanatomy, Vol 3. Elsevier, Amsterdam, pp 141–246
Paxinos G, Watson C (1982) The rat brain in stereotaxic coordinates. Academic Press, New York
Peet MJ, Curry K, Magnusson DSK, McLennan H (1987) The N-methyl-D-aspartate receptor and burst firing of CA1 hippocampal pyramidal neurons. Neuroscience 22: 563–571
Perkins MN, Stone TW (1983) Quinolinic acid: regional variations in neuronal sensitivity. Brain Res 259: 172–176
Reis DJ, Granata AR, Perrone M, Talman WT (1981) Evidence that glutamic acid is the neurotransmitter of baroreceptor afferent terminating in nucleus tractus solitarius (NTS). J Autonom Nerv Syst 3: 321–334
Reynolds RPE, El-Sharkawy TY, Diamant NE (1985) Oesophageal peristaltis in the cat: the role of the central innervation assessed by transient vagal blockade. Can J Physiol Pharmacol 63: 122–130
Reynolds RPE, Effer GW, Bendeck MP (1987) The upper esophageal sphincter in the cat: the role of central innervation assessed by transient vagal blockade. Can J Physiol Pharmacol 65: 96–99
Schaffar N, Pio J, Jean A (1990) Selective retrograde labeling of primary vagal afferent cell-bodies after injection of 3H-D-aspartate into the rat nucleus tractus solitarii. Neurosci Lett 114: 253–258
Sessle BJ, Ball GJ, Lucier GE (1981) Suppressive influences from periaqueductal gray and nucleus raphe magnus on respiration and related reflex activities and on solitary tract neurons, and effect of naloxone. Brain Res 216: 145–161
Siemers ER, Rea MA, Feiten DK, Aprison M (1982) Distribution and uptake of glycine, glutamate and γ-aminobutyric acid in the vagal nuclei and eight other regions of the rat medulla oblongata. Neurochem Res 7: 455–468
Sved AF (1986) Lack of change in high affinity glutamate uptake in nucleus tractus solitarius following removal of the nodose ganglion. Brain Res Bull 16: 325–329
Talman WT, Perrone MH, Reis MJ (1980) Evidence for L-glutamate as the neurotransmitter of baroreceptor afferent nerve fibers. Science 209: 813–814
Van Dongen PAM (1984) Pressure injections of fluid in the nanoliter range via micropipettes. J Neurosci Meth 10: 281–291
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kessler, J.P., Cherkaoui, N., Catalin, D. et al. Swallowing responses induced by microinjection of glutamate and glutamate agonists into the nucleus tractus solitarius of ketamine-anesthetized rats. Exp Brain Res 83, 151–158 (1990). https://doi.org/10.1007/BF00232203
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00232203